摘 要: 为了探究外泌体在卵泡中调控卵母细胞生长发育以及后续受精中的调控作用及机制,首先总结了外泌体的组成和功能,在此基础上选择重点阐述外泌体在卵泡中如何调控卵母细胞与卵泡体细胞的互作机制,然后进一步分析了外泌体介导精卵结合的调控机制,从而得出了外泌体在卵泡中通过其中包含的蛋白和RNA等物质调控卵母细胞生长发育的结论,建议在研究卵母细胞生长发育调控的研究方面应该重视对于外泌体的研究。最后指出了外泌体研究中存在的问题。
关键词: 外泌体; 卵母细胞; 卵泡; 外囊泡; 卵丘细胞;
Abstract: The objectives are to explore exosomes (EXs) regulating oocyte development in the ovarian follicle, and the regulation and mechanism in the subsequent fertilization. Firstly, the composition and function of EXs were reviewed. On this basis, we discussed how EXs regulated the interaction mechanism of oocytes and follicle somatic cells in follicles. And then, we further analyzed the regulation mechanism of EXs mediating sperm-egg binding. Thus, the conclusion was obtained, indicating that EXs regulated the oocyte growth and development through the protein and RNA contained in the follicles, and the research on the regulation of oocyte growth and development should be focused on the study of EXs. In the end, we pointed out problems in EXs research.
Keyword: exosomes; oocyte; ovarian follicle; extracellular vesicles; cumulus cells;
0、 引言
细胞间通信是多细胞生物体的必要标志,并且可以通过直接细胞-细胞接触或分泌的分子的转移来介导。在最近二十年中,出现了涉及细胞外囊泡 (Extracellular Vesicles, EVs) 的细胞间转移的第三种细胞间通讯机制,由细胞释放的各种具有膜结构的囊泡结构统称,这些囊泡的直径可以从30~40 nm到8~9μm[1,2]。是尽管凋亡小体在细胞凋亡过程中的释放早已为人所知[3],但是完全健康的细胞也从其质膜上脱落囊泡的事实最近才被认识。其中外泌体 (Exosomes, EXs) 的大小比较统一,一般在30~100 nm之间,一般认为,EXs通过与质膜融合的方式外排出去,与一般的细胞外囊泡不同。近来研究发现,在马[4]和不同年龄的人[5]卵泡液中,存在这种EXs,如多囊卵巢综合症的病人的卵泡液中就存在[2];后来在奶牛的某些阶段的卵泡液中也存在[1]。EXs在细胞与细胞之间的联系方面有着非常重要的作用,这种细胞之间的联系一般以细胞表面表达的配体直接链接或通过转运生物分子的方式进行。EXs内部包含有蛋白、便于识别靶细胞的细胞识别分子[6]。它们是编码和非编码RNA的天然运输者[7],而且这种运输可以通过血液循环系统长距离作用于靶细胞,并且调控靶细胞的转录和翻译过程[4,7,8]。这些研究同样也说明,其EXs包含独特的方式来转运蛋白或者具有基因调控功能的RNA (mRNA和miRNA) 。细胞外囊泡在临床医学中也有十分光明的应用前景,主要是因为它们含有丰富的生物标志物,可用于监测临床状态,治疗反应,疾病进展等,同时由于它们具有递送生物分子的功能,因此它们还有发展成临床药物递送载体的潜力[9,10,11]。
下面就EXs在卵泡中调控卵母细胞发育的研究进展予以综述,分别从EXs组成及功能、介导体细胞与卵母细胞作用和调控卵母细胞受精等方面予以阐述,期望对于该领域的研究有一定的意义。
1、 EXs组成及功能简介
已有研究发现miRNAs不仅存在与细胞中,而且在不同的流体介质中也大量存在如血浆、血清、尿液、唾液、牛奶和精液中,这些存在的miRNAs一般称之为细胞外miRNAs或循环miRNAs[12,13]。而且有研究发现,这些细胞外miRNAs在核糖核酸酶活性很高的血浆中大量并且稳定的存在[12],由此推测可能这些miRNA存在能够躲避这些外在环境影响的某种保护装置。进一步的研究发现,这些保护装置就是前面提到的不同的流体介质中存在的EXs[14]。EVs通常是细胞持续分泌或在某一刺激条件下产生的几种不同的囊泡结构,可以分为外泌体 (Exosomes, EXs) 、微囊泡 (Microvesicles, MVs) 、凋亡囊泡 (Apoptotic vesicles, AVs) 和病理碎片 (Necrotic debris, NDs) [3,15,16]。一般EVs按照其大小进行分类,EXs (30~100 nm) 、MVs (100~1000 nm) 和AVs (1~5 mm) [3];里面包含有大量的生物活性分子物质如蛋白,mRNA和miRNA,这种结构可以可以从细胞里向外通过从细胞质膜以出芽和裂变的方式释放出来,发挥其生物功能[17]。尤其是外泌体,它来自管腔内的多囊小体 (multivesicular bodies, MVBs) 内陷形成的,然后与质膜融合外排,以向外运输载体的方式释放到细胞[18,19]。值得注意的是外泌小体和微囊泡与细胞膜融合释放的方式是不是一样,到目前为止还不清楚。为方便后面的表述,把分泌到细胞外的分泌小体称之为“外泌体”,这种EXs更加小,一般在30~100 nm之间[7]。
不仅这些不同的EVs的大小不同,而且其包含的蛋白成分和构成也大不相同。微囊泡是在细胞守到激活或应激刺激下直接由于质膜内陷包裹形成囊状结构释放出来,但是外泌体是在细胞内的内质网内在囊状结构与之在分离开,然后通过转运通过细胞质膜释放出来,而凋亡囊泡是在细胞收到凋亡信号的刺激后以细胞出泡的方式释放出来[16,20,21]。目前还没有特殊的标记可以区分这些不同类型的EVs。微囊泡中一般含有与转运有关的内质网分选复合体 (endosomalsorting complexes required for transport, ESCRT) -Alix、TSG101蛋白和四跨膜蛋白超家族如CD63, CD81和CD9,但是这些蛋白也不只是独一无二在微囊泡中表达,利用这些蛋白加上大小的标志基本可以鉴别微囊泡结构[8,15,17,22]。目前定量和定性研究EVs的方法主要有以下集几种:酶联相关免疫吸附实验、流式细胞技术、电子显微镜技术和WB技术,近来,很多新技术也应用在EVs的研究,如冷冻电子显微镜技术和纳米颗粒追踪技术等[16,23]。
2、 EXs介导卵泡体细胞与卵母细胞的相互作用
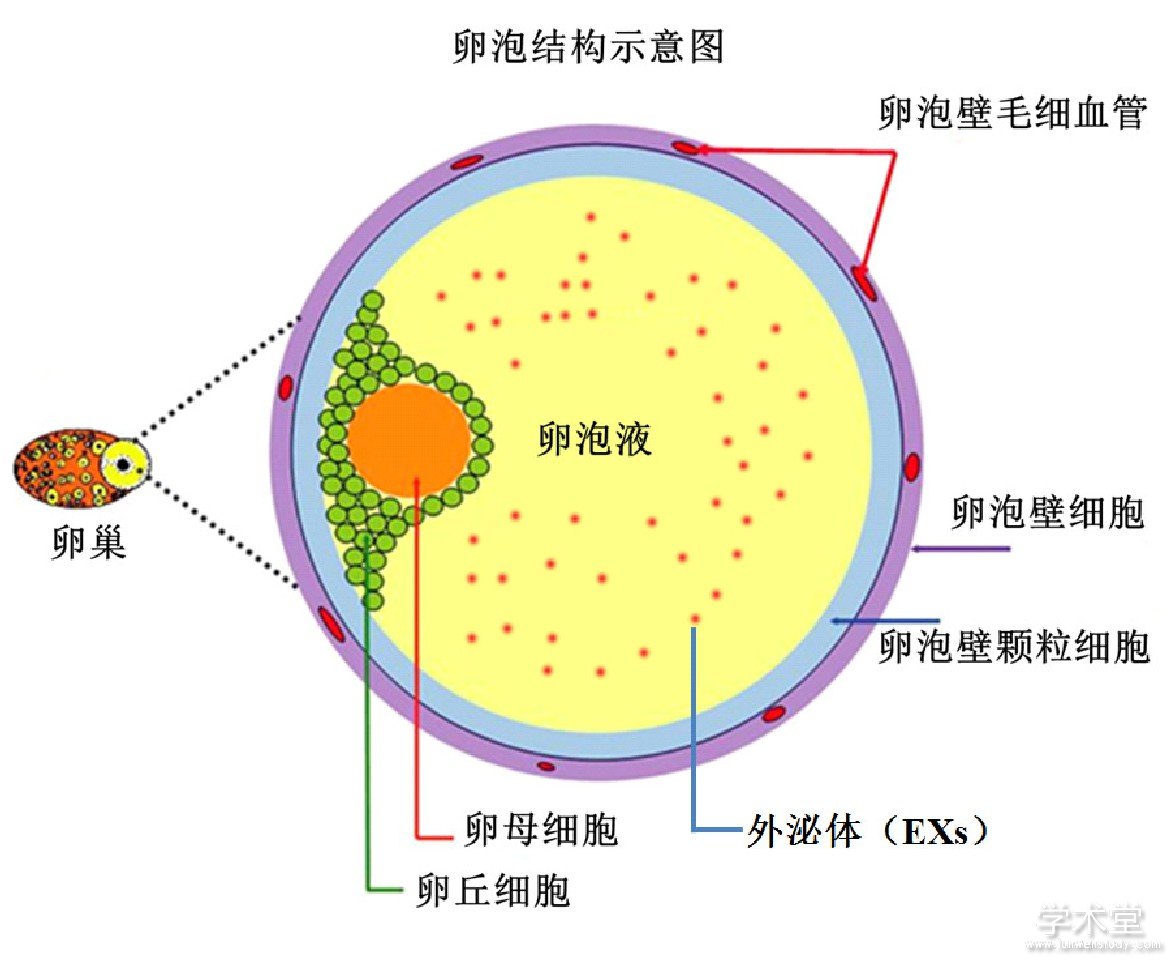
卵泡从外到内的结构简述如下:卵泡壁细胞,其上被缠绕毛细血管;在卵泡壁内周上是颗粒细胞;然后是环绕在卵母细胞上的卵丘细胞;最里面就是卵母细胞[24,25,26,27,28,29]。在卵母细胞上卵丘细胞与卵泡壁上的颗粒细胞之间的卵泡腔中,存在大量的卵泡液。而且卵泡液的数量随着卵泡的生长不断增加,同时可以与血液进行物质的交换,尤其与其围绕的颗粒细胞和卵丘细胞发生大量的物质交换[30,31,32]。目前研究发现,主要以EXs为载体进行细胞信号的传导,调控卵母细胞的发育成熟[33]。其卵泡中各种细胞之间的互相作用关系可见下图(图1)。
卵泡中存在卵母细胞与卵丘细胞、颗粒细胞和卵泡壁颗粒细胞有着密切联系,这些联系对于卵母细胞的生长发育至关重要[24,25,26,27,28,29,34,35,36],尤其是包围在卵母细胞周围的各种体细胞其合成的相关激素等物质会以EXs的形式包裹以来,运输通过卵泡液的特殊液体环境到达作用的靶标-卵母细胞,发挥相应的调控作用,比如促进卵母细胞的发育成熟和启动减数分裂[37,38]。尤其在卵母细胞生长过程中,EXs是如何协调各种不同细胞之间的联系对于卵母细胞的生长、成熟和后续排卵以及受精有着非常重要的作用。其中,各种内分泌激素如FSH和LH对于卵泡从腔前卵泡生长到有腔卵泡阶段,起着必不可少的作用。同时,来源卵母细胞的细胞因子如GDF-9和BMP-15,也能影响临近的颗粒细胞生长发育[39,40],尤其是卵泡腔中积累的卵泡液来源于卵泡壁上血管里面的血液系统,这些卵泡液把这些细胞彼此分隔开[28,31]。这种特殊的卵泡结构,通过EXs的介导,使得卵泡可以与通过毛细血管与血液系统发生充分的物质交换,同时这些围绕的体细胞和卵母细胞分泌的各种物质也可以进入血液系统,发挥其精确调控功能,使得卵母细胞能够在其生长历程中按照预定的方式进行发育[41,42]。可见卵泡这个特殊的复杂结构中EXs为卵母细胞的成熟发育提供了独一无二的条件。
图1 卵泡体细胞细胞与卵母细胞的互作结构图
3、 EXs对卵母细胞受精的调控作用
研究发现,EXs对于细胞之间的联系发挥重要的作用,可以彼此之间传递蛋白质、RNA和miRNA等分子,通过运输可以到达作用的靶细胞[17]。在卵泡生长伴随的卵母细胞成熟的过程中,miRNAs可以以一种时空作用的方式协调调节各种基因的表达[43]。同时,细胞外的miRNAs也在一些没有EXs的流体介质中发现了,如AGO2 (Argonaute 2) ,它是miRNA诱导基因沉默的效应器,会引起细胞中mRNA表达沉默[44]。
精子与卵母细胞的融合是受精的一个必要环节。其中,CD9在受精中发挥重要的作用,有研究表明CD-9敲除小鼠的卵母细胞不能与精子完成受精过程[45,46]。利用转基因小鼠作为动物模型进行实验,发现卵母细胞通过类似于“外分泌体”的囊泡方式把CD9转移到精子的头部[47]。这些富含CD9的EXs主要定位在卵母细胞的卵周膜 (Perivitelline space, PVS) 的位置,直径大小在50~250 nm,而且还表达外分泌体的2个标记蛋白-神经节苷脂GM3和热休克蛋白90[24,25,26]。同时也有研究发现,在卵母细胞的PVS区域存在大小50~150 nm的EVs,这些EXs与CD9密切相关[48]。但是有关积累在PVS中的EXs是由于CD9突变引起的,还是CD9的突变依赖于卵母细胞分泌的EXs不是很清楚[47,48]。总之,这些在不同流体介质中EXs含有的各种miRNAs,有可能为揭示各种不同生理或病理状态下的各种生物标记,为后续的疾病早诊断和预防提供了新的思路[49]。
4、 展望
目前,已发现所有细胞均可释放包括EXs在内的各种细胞外囊泡,可以向近端和远端细胞递送脂质、蛋白质和核酸,发挥重要的生物学功能。但是有感EXs的研究也还存在以下的问题:(1) EXs鉴定的颗粒大小标准不统一,其范围只是报道在30~100 nm之间;(2)有关提取EXs的方法主要采取传统差速离心的方法,难以保证每次提取的获取EXs的成功率,而且难以做到百分之百的纯度;(3)有关后续EXs的分子鉴定标记也没有统一;(4)有关EXs后续进一步RNA实验和蛋白检测等实验方法没有建立,需要进一步的研究予以拓展。尽管还存在上述的问题,但是目前关EXs在疾病的液体分子检测和诊断方面已经取得了很大的进展,尤其是在疾病液体诊断和靶向药物治疗等方面[50],相信在不久的将来,将会进一步掀起EXs在各个研究领域的高潮。当然在有关卵母卵母细胞发育的研究,也可以利用EXs作为载体进行离体卵泡体外培养研究,进一步阐述卵泡中体细胞与卵母细胞的互相作用机制。
参考文献
[1] Sohel M M H, Hoelker M, Noferesti S S, et al. Exosomal and nonexosomal transport of extra-cellular microRNAs in follicular fluid:implications for bovine oocyte developmental competence[J]. PloS one, 2014, 8 (11) :e78505.
[2] Roth L W, Mccallie B, Alvero R, et al. Altered microRNA and gene expression in the follicular fluid of women with polycystic ovary syndrome[J]. Journal of assisted reproduction and genetics, 2014, 31 (3) :355-362.
[3] Raposo G, Stoorvogel W. Extracellular vesicles:exosomes, microvesicles, and friends[J]. The Journal of cell biology, 2013, 200 (4) :373-383.
[4] Silveira J C D, Veeramachaneni D N R, Winger Q A, et al. Cellsecreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins:a possible new form of cell communication within the ovarian follicle[J]. Biology of reproduction, 2012, 86 (3) :1-10.
[5] Santonocito M, Vento M, Guglielmino M R, et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid:bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation[J].Fertility and sterility, 2014, 102 (6) :1751-1761.
[6] María M, Cristina G V, Carolina V B, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells[J]. Nature communications, 2011, 2:282.
[7] Valadi H, Ekstr?m K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nature cell biology, 2007, 9 (6) :654-659.
[8] Pegtel D M, Cosmopoulos K, Thorleylawson D A, et al. Functional delivery of viral miRNAs via exosomes[J]. Proceedings of the National Academy of Sciences, 2010, 107 (14) :6328-6333.
[9] Lim A K, Knowles B B. Controlling endogenous retroviruses and their chimeric transcripts during natural reprogramming in the oocyte[J]. Journal of Infectious Diseases, 2015, 212 (suppl 1) :S47-S51.
[10] Jaitin D A, Kenigsberg E, Keren-Shaul H, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types[J] Science, 2014. 343 (6172) :776-779.
[11] Islam S, Zeisel A, Joost S, et al. Quantitative single-cell RNA-seq with unique molecular identifiers[J]. Nature methods, 2014, 11 (2) :163-166.
[12] Mitchell P S, Parkin R K, Kroh E M, et al. Circulating microRNAs as stable blood-based markers for cancer detection[J]. Proceedings of the National Academy of Sciences, 2008, 105 (30) :10513-10518.
[13] Weber J A, Baxter D H, Zhang S, et al. The microRNA spectrum in12 body fluids[J]. Clinical chemistry, 2010, 56 (11) :1733-1741.
[14] Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication[J]. Current opinion in cell biology, 2009, 21 (4) :575-581.
[15] Cronqvist T, Salje K, Familari M, et al. Syncytiotrophoblast vesicles show altered micro-RNA and haemoglobin content after exvivo perfusion of placentas with haemoglobin to mimic preeclampsia[J]. PloS one, 2014, 9 (2) :e90020.
[16] Pol E, Coumans F, Varga Z, et al. Innovation in detection of microparticles and exosomes[J]. Journal of Thrombosis and Haemostasis, 2013, 11 (s1) :36-45.
[17] Muralidharan-Chari V, Clancy J W, Sedgwick A, et al.Microvesicles:mediators of extracellular communication during cancer progression[J]. J Cell Sci, 2010, 123 (10) :1603-1611.
[18] Vlassov A V, Magdaleno S, Setterquist R, et al. Exosomes:current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials[J]. Biochimica et Biophysica Acta (BBA) -General Subjects, 2012, 1820 (7) :940-948.
[19] Simpson R J, Lim J W E, Moritz R L, et al. Exosomes:proteomic insights and diagnostic potential[J]. Expert review of proteomics, 2009, 6 (3) :267-283.
[20] Hu J H, Qu-Lei W Z, Zhao W, et al. Research on the Effects of Different Cryopreservated Mediums on Shanbei Cashmere Goat Semen Cryopreservation[J]. Journal of Animal and Veterinary Advances, 2013, 12 (5) :640-643.
[21] Chew T G, Lorthongpanich C, Ang W X, et al. Symmetric cell division of the mouse zygote requires an actin network[J].Cytoskeleton, 2012, 69 (12) :1040-1046.
[22] Tashiro F, Kanai-zuma M, Miyazaki S, et al. Maternal-ffect gene Ces5/Ooep/Moep19/Floped is essential for oocyte cytoplasmic lattice formation and embryonic development at the maternal zygotic stage transition[J]. Genes to Cells, 2010, 15 (8) :813-828.
[23] Witwer K W, Buzas E I, Bemis L T, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research[J]. Journal of extracellular vesicles, 2013, 2:20360-20381.
[24] Yurttas P, Vitale A M, Fitzhenry R J, et al. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo[J]. Development, 2008, 135 (15) :2627-2636.
[25] Ohsugi M, Zheng P, Baibakov B, et al. Maternally derived FILIAMATER complex localizes asymmetrically in cleavage-stage mouse embryos[J]. Development, 2008. 135 (2) :259-269.
[26] Li L, Baibakov B, Dean J. A subcortical maternal complex essential for preimplantation mouse embryogenesis[J]. Developmental cell, 2008. 15 (3) :416-425.
[27] Diaz F J, Wigglesworth K, Eppig J J. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice[J].Developmental biology, 2007, 305 (1) :300-311.
[28] Conti M, Hsieh M, Park J Y, et al. Role of the epidermal growth factor network in ovarian follicles[J]. Molecular Endocrinology, 2006, 20 (4) :715-723.
[29] Wright PW, Bolling LC, Calvert ME, et al. ePAD, an oocyte and early embryo-abundant peptidylarginine deiminase-like protein that localizes to egg cytoplasmic sheets[J]. Developmental biology, 2003, 256 (1) :74-89.
[30] Kim B, Kan R, Anguish L, et al. Potential role for MATER in cytoplasmic lattice formation in murine oocytes[J]. PloS one, 2010.5 (9) :e12587.
[31] Carletti M Z, Fiedler S D, Christenson L K. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells[J]. Biology of reproduction, 2010, 83 (2) :286-295.
[32] Zheng J. Dean, Role of Filia, a maternal effect gene, in maintaining euploidy during cleavage-stage mouse embryogenesis[J].Proceedings of the National Academy of Sciences, 2009, 106 (18) :7473-7478.
[33] Coticchio G, Dal Canto M, Renzini M M, et al. Oocyte maturation:gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization[J]. Human reproduction update, 2015, 21 (4) :427-454.
[34] Khamsi F, Roberge S. Granulosa cells of the cumulus oophorus are different from mural granulosa cells in their response to gonadotrophins and IGF-I[J]. Journal of endocrinology, 2001, 170 (3) :565-573.
[35] Tong Z B, Nelson L M. A mouse gene encoding an oocyte antigen associated with autoimmune premature ovarian failure[J].Endocrinology, 1999, 140 (8) :3720-3726.
[36] Kumar T R, Wang Y, Lu N, et al. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility[J].Nature genetics, 1997, 15 (2) :201-204.
[37] Matzuk M M, Burns K H, Viveiros M M, et al. Intercellular communication in the mammalian ovary:oocytes carry the conversation[J]. Science, 2002, 296 (5576) :2178-2180.
[38] Wigglesworth K, Lee K B, Emori C, et al. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles[J]. Biology of reproduction, 2015, 92 (1) :23-37.
[39] Appeltant R, Somfai T, Nakai M, et al. Interactions between oocytes and cumulus cells during in vitro maturation of porcine cumulus-oocyte complexes in a chemically defined medium:effect of denuded oocytes on cumulus expansion and oocyte maturation[J]. Theriogenology, 2015, 83 (4) :567-576.
[40] Park J Y, Su Y Q, Ariga M, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle[J]. Science, 2004, 303 (5658) :682-684.
[41] Navakanitworakul R, Hung W-T, Gunewardena S, et al.Characterization and Small RNA Content of Extracellular Vesicles in Follicular Fluid of Developing Bovine Antral Follicles[J].Scientific reports, 2016, 6:25486-25499.
[42] Cakmak H, Franciosi F, Zamah A M, et al. Dynamic secretion during meiotic reentry integrates the function of the oocyte and cumulus cells[J]. Proceedings of the National Academy of Sciences, 2016, 113 (9) :2424-2429.
[43] Hossain M M, Sohel M M H, Schellander K, et al. Characterization and importance of microRNAs in mammalian gonadal functions[J].Cell and tissue research, 2012, 349 (3) :679-690.
[44] Arroyo J D, Chevillet J R, Kroh E M, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma[J]. Proceedings of the National Academy of Sciences, 2011, 108 (12) :5003-5008.
[45] Kaji K, Oda S, Shikano T, Ohnuki T, et al. The gamete fusion process is defective in eggs of Cd9-deficient mice[J]. Nature genetics, 2000, 24 (3) :279-282.
[46] Miyado K, Yamada G, Yamada S, et al. Requirement of CD9 on the egg plasma membrane for fertilization[J]. Science, 2000, 287 (5451) :321-324.
[47] Miyado K, Yoshida K, Yamagata K, et al. The fusing ability of sperm is bestowed by CD9-containing vesicles released from eggs in mice[J]. Proceedings of the National Academy of Sciences, 2008, 105 (35) :12921-12926.
[48] Barraud-Lange V, Boissonnas C C, Serres C, et al. Membrane transfer from oocyte to sperm occurs in two CD9-independent ways that do not supply the fertilising ability of Cd9-deleted oocytes[J].Reproduction, 2009, 144 (1) :53-66.
[49] Cortez MA, Welsh JW, Calin GA. Circulating microRNAs as noninvasive biomarkers in breast cancer[J]. Recent Results Cancer Res., 2012, 195:151-161.
[50] Yang Z, Xie J, Zhu J, et al. Functional exosome-mimic for delivery of siRNA to cancer:in vitro and in vivo evaluation[J]. Journal of Controlled Release, 2016, 243:160-171.
哺乳动物卵泡发育分子机制的相关研究已取得了巨大的成果。目前, 已经证明了许多信号通路在卵泡生长发育过程中起着非常重要的作用, 并且其中一些信号通路的研究已经非常透彻。...