求助主题我需要PMID:32695762的文献
需求说明PMID:32695762
标题:Construction of a Robust Sphingomonas sp. Strain for Welan Gum Production via the ex
求助时间2020-11-26 16:05
您好!根据您的需求,学术堂为你查找到《Construction of a Robust Sphingomonas sp. Strain for Welan Gum Production via the ex<x>pression of Global Transc<x>riptional Regulator IrrE》全文,下载附件可以查看全文。下面的部分内容展示:
Xiaoliu Liu1,2, Ming Zhao1,3, Zheng Xu1,3, Hong Xu1,3,4* and Sha Li1,3*
1State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing, China,2College of Bioscience and Engineering,Hebei University of Economics and Business, Shijiazhuang, China,3College of Food Science and Light Industry, Nanjing Tech University, Nanjing, China,4Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing,China
Welan gum is a widely used microbial polysaccharide produced by Sphingomonassp. However, an important factor hindering the expansion of its production is themaladaptation of strain to fermentation conditions. In this work, the global transcriptionalregulator gene irre was selected as a stress-resistant element. And it was integrated intothe site of the genomic carotene synthesis key enzyme gene crtB to construct a robustcarotenoid-free welan gum producing strain. Fermentation with the recombinant straineffectively reduced the ethanol consumption and pigment content in the product. Thetolerance temperature increased by 10?C without the need for controlling the pH. Underthis fermentation condition, welan gum concentration could still reach 20.26 ± 0.25 g/L,which was 187.38% higher than that of the wild-type strain (7.05 ± 0.15 g/L)。
Transcriptome analysis showed that with the control of IrrE, more than 1000 genesthat are involved in multiple pathways, including two-component system, bacterialchemotaxis, flagellar assembly, and cell cycle, exhibited changes at the transcriptionallevel and jointly allowed the strain to protect against environmental stresses.
Keywords: welan gum, IrrE, robust, carotenoid free, RNA-seq .
INTRODUCTION.
Sphingomonas sp. is a promising producer of gellan gum polysaccharide, such as gellan gum, welangum, rhamsan gum, and so on. Welan gum is an important member of gellan gum family that iswidely used in food, medicine, concrete additives, and oil recovery because of its high and stableviscosity in aqueous solution over a broad range of temperature and pH (Zhu et al., 2014a; Ai et al.,2015; Liu et al., 2017)。 However, many restrictive factors in gellan gum polysaccharide productionlimit its yield and large-scale application.
The main reason lies in the poor environment toleranceof the Sphingomonas sp. strain. For welan gum production,heat from mechanical agitation and microbial metabolism (Zhuet al., 2014a), high viscosity and weak acidity from welan gumaccumulation (Li et al., 2011a), and pigment from carotenoidsynthesis (Zhang et al., 2016) result in the large demand ofcooling water, alkali binding agent, strong ventilation or agitationfor fermentation, and alcohol for polysaccharide purification,thereby causing high energy consumption and cost.
Robustness toward industrial conditions is a key featurein engineering microorganisms for industrial metaboliteproduction (Marc et al., 2017)。 For the past few years, mutationbreeding, adaptive laboratory evolution, and resequencingexperiments have been used to obtain advanced industrial strains(Barrick and Lenski, 2013)。 Zhu et al. (2014a) obtaineda mutant S. sp. by atmospheric and room-temperatureplasma-induced mutation, and the mutant could grow wellat 37?C. Nevertheless, these approaches have difficult inmutation direction grasping, beneficial combinatorial landscapeoccurrence, target strain screening, and good traits passing on(Blooma and Arnoldb, 2009)。 Synthetic biological technologies,especially metabolic engineering, are used to improve theperformance of industrial microorganisms (Chae et al., 2017)。
However, most cellular phenotypes are controlled by severalgenes. Current metabolic engineering approaches are limited bymultiple gene modification (Alper et al., 2005)。
Therefore, approaches such as generating globaltranscriptional regulators and heat shock proteins to achievemultiple regulatory have been developed (Lin et al., 2013;Mukhopadhyay, 2015)。 Transcriptional regulators play crucialroles in microbial growth and metabolism by regulating geneexpression, repairing DNA or protein, and promoting theformation of biofilms (Alper and Stephanopoulos, 2007; Zhuet al., 2016)。 For example, mutational housekeeping σ70 factor(Gruber and Gross, 2003) enhances the ethanol and SDStolerance of Escherichia coli (Santos and Stephanopoulos, 2008)。
Over-expressed transcriptional activator encoding gene HAA1increases the acetic acid tolerance of Saccharomyces cerevisiae(Tanaka et al., 2012)。 Exogenous DNA repair-related proteinIrrE improves the radiation (Gao et al., 2003), osmosis, heat,and oxidation tolerance (Jie et al., 2009) of E. coli and thealcohol, heat, and osmosis tolerance of Zymomonas mobilis(Chen, 2010)。 Heat shock proteins are involved in importantphysiological processes, such as proper folding, aggregation,transport, and signaling of proteins (Lindquist, 1992; Yuet al., 2015)。 They are synthesized under stress conditions toguarantee cell survival. GroESL overexpression endows E. coliwith a remarkable tolerance to n-butanol, i-butanol, 2-butanol,1,2,4-butanetriol, and ethanol (Zingaro and Terry Papoutsakis,2013) and imparts Clostridium. tyrobutyricum with toleranceto butyric acid (Suo et al., 2017)。 The heteroexpression ofTamarix hispida Hsp18.3 improves the resistance of yeast to heat,osmosis, and heavy metals (Gao et al., 2012)。 Nevertheless, nostrategy has been developed to increase the resistance of welangum-producing strains.
In addition to poor resistance to environmental stress, thesynthesis of carotenoids accompanying welan gum production isanother issue that should be considered. Carotenoids are derivedfrom the isoprenoid or steroidal pathway with isopentenylpyrophosphate (IPP) as a precursor. In fungi, archaea, and mosteukaryotes, IPP is synthesized by acetyl CoA via the mevalonatepathway. Meanwhile, in most bacteria and algae, IPP is producedby pyruvate and glyceraldehyde-3-phosphate via the methylerythritol pathway (Armstrong et al., 1990; Preejith et al., 1996)。
Wu et al. analyzed the carotenoid synthesis pathway in thegellen gum-producing strain Sphingomonas eloda ATCC31461.
The key enzymes of this strain include phytoene synthase,phytoene dehydrogenase, lycopene cyclase, 2,2'-β-ionone ringhydroxylase, and 3,3'-β- ionone ring hydroxylase, and the codinggenes are crtB, crtI, crtY, crtG, and crtZ, respectively (Wuet al., 2011)。 Similar results were also confirmed in anotherwelan gum-producing strain, Sphingomonas sp. ATCC31555(Zhang et al., 2016)。 These results provided a useful referencefor our research.
In the present work, heat shock proteins and globaltranscriptional regular factor from extremophile wereexpressed in the welan gum producing-strain S. sp. NX-3to enhance its complex phenotypes. The selected stressresistant element was then inserted into the key enzymegene site of the carotene synthesis pathway in the genome,thereby constructing a pigment production defect strainwith multiplex stress resistant. Subsequently, fermentationperformance and genome-wide transcriptional analyses wereconducted to investigate the external and internal influencesof the metabolic modification above. Although we focusedon the robustness of the welan gum-producing strain,the concepts discussed herein are also applicable to otherindustrial strains.
MATERIALS AND METHODS.
Strains and Plasmids.
Relevant characteristics of plasmids and strains are listed inTable 1. E. coli DH5α harboring recombinant pBBR1MCS-5plasmids and E. coli. S17-1 harboring recombinant pJQ200SKplasmids were used as the donors in conjugal transfer. E. coliHB101 (pRK600) was served as the helper strain in conjugaltransfer. And, S. sp. NX-3 and its derivatives were employed asthe acceptors in this study.
Recombinant Plasmids Construction
In-fusion cloning was used to construct the recombinantplasmids. The primers used in this study are listed in Supplementary Table S1.
For the construction of the recombinant Sphingomonasexpression vector pBBR-groes, the gene groes (Sequence ID:AE008691.1) carrying homologous arms on both ends wasamplified using the primer pair pET-groes-F/pET-groes-R withthe genome of Thermoanaerobacter tengcongensis MB4 as atemplate. After purification and sequencing, the fragment abovewas cloned into linearized vector pET28a with the restriction sitesof Nco I and Not I, forming plasmid pET-groes. The recombinantpET-groes plasmid was used as a template to amplify the gene groes carrying the T7 promoter, which was purified, sequenced,and ligated into the linearized broad host plasmid pBBR1MCS5 with Hind III/BamH I to obtain recombinant Sphingomonasexpression vector pBBR-groes. The plasmid construction andgene deletion processes are shown in Supplementary Figures S1,S2. This method is also applicable to the construction of pBBRgroel (Sequence ID for groel was AP008226.1) and pBBR-irre(Sequence ID for irre was CP015081.1)。
For the construction of the crtB gene deletion vector, theupstream and downstream flanking sequences of the crtB genewere amplified using the degenerate primer pairs crtB-LF/crtBLR and crtB-RF/crtB-RR. The two fragments were joined byoverlap-PCR and cloned into the lined suicide vector pJQ200SK(BamH I/Xba I), resulting in the crtB gene deletion vector pJQ1crtB. This method is also applicable to the construction of irregene insertion vector pJQ-1crtB-irre.
Conjugation Transfer.
Strains and plasmids used in this study were shown in the Table 1.Recombinant Sphingomonas expression vectors and crtB genedeletion vector pJQ-1crtB were transformed into E. coli DH5αand E. coli S17-1, respectively, yielded the donors in conjugaltransfer. The specific process of conjugation transfer method wasdescribed in the previous study (Liu et al., 2017)。 The coloniesthat developed on the screening medium were candidates ofS. sp. harboring expression vectors. Two steps of homologousrecombination were needed to obtain the crtB knockout andirre insertion strains. Thus, the colonies that developed onthe screening medium should be cultured in seed medium forSphingomonas containing 5% (v/v) sucrose overnight and theculture solution was then diluted and spread at the screeningmedium containing 5% (v/v) for the selection of colorless clones.
Media and Culture Conditions.
LB medium (g/L): peptone 10, yeast powder 5, NaCl 10 (solid plus1.5% agar)。 The final concentration of each antibiotic in the E. coliresistant medium was (?g/mL): kanamycin 25, gentamicin 50.
Agar medium for S. sp. (g/L): glucose, 10; beef extract, 3;peptone, 10; NaCl, 5; and agar, 20, pH 7.2-7.4. Seed medium for S.sp. (g/L): glucose, 20; yeast extract, 1; peptone, 3; K2HPO4·3H2O, 2; and MgSO4, 0.1, pH 7.2-7.4. Fermentation medium for S.sp. (g/L): glucose, 50; yeast extract, 8; K2HPO4·3H2O, 3; andMgSO4, 0.4, pH7.2-7.4 (Zhu et al., 2014a,b)。 Conjugative transferscreening medium (g/L): glucose 20, yeast extract 1, peptone 3,K2HPO4·3H2O 2, MgSO4 0.1, pH 7.2-7.4. Final concentration ofeach antibiotic (?g/mL): gentamicin 50, streptomycin 100. Finalconcentration of isopropyl-β-d-thiogalactoside (?g/mL): 0.4, andadded at the initial stage of fermentation.
For flask cultivation, strains were streaked out from frozenglycerol stocks onto plates containing the activation medium andincubated at 30?C for 36 h. One loop of colonies was inoculatedin 100 mL seed medium in a 500 mL flask. After 16h, the seedculture was inoculated into 100 mL fermentation medium in a500 mL flask, and the initial optical density at 600 nm (OD600)was adjusted to 0.2. The culture was incubated at 30?C and200 rpm for 66 h.
For fermentor cultivation, 100 mL seed culture was firstprepared in 500 mL flasks at 30?C for 16 h, then it was transferredto a 7.5 L fermentor (New Brunswick Scientific BioFlo 110,United States) containing 4.5 L fermentation medium, and thepH was adjusted to 7.4 with 3 M NaOH and the media wereautoclaved prior to use. The aeration rate was maintained at 1.0vvm with an agitation speed of 600 rpm for 60 h. To test the effectof the stress-resistant elements, different temperature gradientsbetween 30 and 43?C, and different pH gradients between 4 and10 might be used.
Analytical Methods
Dry cell weight (DCW) was measured to reflect the cell growth,which was determined as previously reported (Li et al., 2011a)。Glucose content was measured using a biosensor equippedwith a glucose oxidase electrode (SBA-40C, Shandong Academyof Sciences, China)。 Culture broth viscosity and rheologicalbehavior of welan gum solutions were measured by a rotationalviscometer (NDJ-1, Shanghai Hengping Scientific InstrumentCompany, China) with rotor No. 4 at 60 rpm (Ai et al., 2015;Li et al., 2011b)。 The welan gum production was determinedby isopropanol precipitation and dry weight measurement(Li et al., 2010)。
RNA-Seq Analysis.
Total RNA was extracted in biological triplicates from strainsS. sp. NX-3 and S. sp. NX-R cultured at 30?C and pH7.0 and S. sp. NX-R cultured at 40?C and natural pH for20 h using TaKaRa RNAiso Plus kit. The concentration andpurity of total RNA was determined using NanoDrop, andthe integrity of the RNA was identified using Agilent 2100and simulated electrophoresis gel images. The qualified RNAsamples were sent to the Beijing Genomics Institute (BGI)for library constructing and sequencing. After data filtering,reference genome comparison and gene expression analysis,significant differentially expressed genes were filtered for geneswith log2 fold change > 1 (upregulation) or log2 fold change < -1(downregulation) with p-value and FDR both <0.05 (Tang et al.,2008; Xie et al., 2011)。 Significant differentially expressed geneswere further cluster analyzed and functional enrichment analyzedto determine its most important biochemical metabolic pathwaysand signal transduction pathways.
RESULTS.
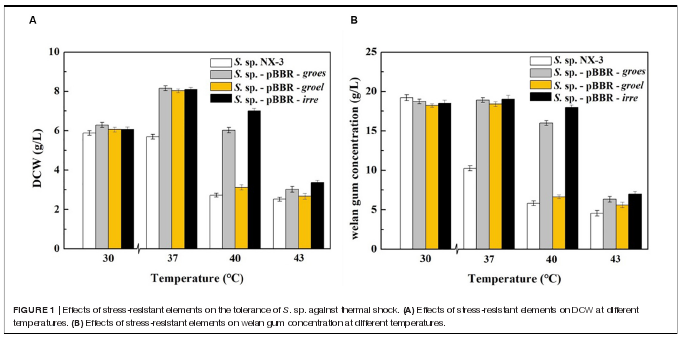
Effects of Stress-Resistant Elements on the Tolerance of S. sp. Against Thermal Shock.
Firstly, stress-resistant elements derived from extremophiles,including heat shock proteins GroES of Thermoanaerobactertengcongensi, GroEL of Thermus thermophilus and globaltranscriptional regulator IrrE of Deinococcus radiodurans, wereheterologously expressed in the welan gum-producing strain S.sp. NX-3 to improve cell tolerance.
With the increase in fermentation temperature from 30?Cto 43?C, both the final DCW and welan gum concentration ofthe control strain decreased (Figure 1)。 The maximum biomassand welan gum concentration at 30?C were 5.89 ± 0.12 g/Land 19.23 ± 0.20 g/L, respectively. Meanwhile, all of therecombination strains showed a good performance at 37?C notonly in maximum biomass but also in maximum welan gumconcentration. At 30?C, the difference between the recombinantand control strains in terms of bacterial biomass and welangum concentration was not significant. When the fermentationtemperature increased to 43?C, the cell growth of S. sp. NX3 was almost stagnant, followed by the cessation of welan gumsynthesis. The expression of the stress-resistant elements didnot have significant positive effects to the results. When thefermentation temperature increased to 40?C, the recombinantstrains S. sp.-pBBR-groes and S. sp.-pBBR-irre showed significantadvantage compared with the control strains, although thebiomass and welan gum concentration at the end of fermentationprocess were slightly lower than those of 37?C. Recombinantstrain harboring global transcriptional regulator IrrE performedwell, with the DCW and welan gum concentration reaching7.00 ± 0.11 g/L and 17.98 ± 0.28 g/L, thereby obtaining1.57- and 2.09-fold increase than those of the control strains(2.72 ± 0.10 g/L and 5.82 ± 0.11 g/L), respectively.
Effects of Stress-Resistant Elements on the Tolerance of S. sp. Against Weak Acidity Shock.
The growth and welan gun production of the reconstructionstrain expressing IrrE under natural pH conditions at thistemperature were investigated. The results were shown inFigure 2. As the fermentation process progressed, the acidicpolysaccharide gradually accumulated, and the final pH of thefermentation broth decreased to approximately 4.2. Under thiscondition, the DCW of S. sp.-pBBR-irre was 6.50 ± 0.12 g/L,which was equal to 95.17% of the outcome of the wildtype strain at optimum fermentation conditions (30?C andpH of 7.0)。 Correspondingly, the welan gum concentrationof the IrrE expressing strain was 20.12 ± 0.20 g/L, whichreached 94.02% of that of the wild-type strain at optimumconditions (21.40 ± 0.30 g/L)。 Meanwhile, the DCW and welan gum concentration of S. sp. NX-3 were 3.02 ± 0.11 g/L and7.00 ± 0.20 g/L, which were only 46.46% and 34.79% of thoseof the IrrE expressing strain, respectively. The expression of theglobal regulatory factor IrrE remarkably improved the resistanceof S. sp. to acidic pH.
In summary, S. sp.-pBBR-irre is suitable for the fermentationprocess at 40?C and natural pH conditions, which can effectivelydecrease the amount of cooling water, remove the use ofneutralizing alkali and pH electrode, simplify the fermentationdevice, and reduce the production cost.
Construction of Carotenoid-Free Welan Gum-Producing Strain.
Comparative genomics was used to evaluate the key genesinvolved in carotenoids synthesis of in S. sp. NX-3. Comparedwith previous studies, S. sp. NX-3 had extremely highhomology with the gellan gum-producing strain Sphingomonaselodea ATCC31461 and another welan gum-producing strainSphingomonas sp. ATCC31555 (95% and 98%, respectively) (Gaiet al., 2011; Wang et al., 2012), whose pigment synthesis pathwayshave been reported. Genes crtB, crtI, crtY, crtG, and crtZ wereamplified using the S. sp. NX-3 genome as a template. Aftersequencing, the gene sequences of 923, 1478, 797, 1157, and501 bp were obtained, which were consistent with the prospectivelength. Through BLAST alignment, the homology between thegenes in S. sp. NX-3 and S. elodea ATCC31461 and S. sp.ATCC31555 reached 99%. Therefore, S. sp. NX-3 may have thesame key genes for pigment synthesis.
Suicide vector with the up- and downstream homologousarms of crtB was constructed, and strain S. sp.-1crtBwas obtained after conjugation transfer and homologousrecombination (Figure 3A)。 The original (Figure 3B-0) andcrtB deletion bacteria (Figure 3B-1) were inoculated onto thesame plate to compare the colonial morphology. As shown inthe figure, before and after the crtB gene knockout, the colonialmorphology did not change significantly, both were round, andthe edges were smooth. However, after crtB gene deletion, thecolonies appeared colorless. The shake flask fermentation results(Figure 3C) also showed that the fermentation broth turnedwhite after the crtB gene was knocked out. This result furtherindicated that the crtB gene is one of the key genes of the pigmentsynthesis pathway in S. sp. NX-3, and knocking crtB gene caneffectively block the production of yellow pigment in S. sp.
Welan gum production at 30?C and pH of 7.2-7.4 andethanol consumption before and after crtB deletion are shownin Table 2. The welan gum concentration of S. sp.-1crtB wasslightly higher than that of S. sp. NX-3, possibly because crtBknockout blocked the synthesis of carotenoids and made themore metabolic flow turned to the welan gum synthesis pathway.
Ethanol consumption, ethanol cost, and product color were alsocompared. The results indicated that crtB deletion can reducethe ethanol cost by 36.9% in the purification process, and theproduct color was significantly better than the product beforeknockout. Lower pigment content in waste ethanol was alsobeneficial to its reuse.
Construction of Multi-Resistance Carotenoid-Free Welan Gum-Producing Strain.
The results of See section “Effects of Stress-Resistant Elementson the Tolerance of S. sp. Against Thermal Shock” showthat the expression of the global transcriptional regulator IrrEcan effectively improve the tolerance of the strain to hightemperature and acidic pH. However, the plasmid expressionexhibited poor stability, and antibiotics required extremophiles,thereby resulting in high cost and disadvantages in later gene manipulation. To overcome these defects, S. sp. NX-R strainwas constructed by inserting the irre gene into the crtB siteof the S. sp. NX-3 genome, and a band with a molecularmass of approximately 32 kDa was visualized from recombinantstrain after induction (Figure 4A)。 The fermentation of S.sp. NX-3, S. sp.-1crtB, and S. sp. NX-R was compared under different fermentation conditions. At 30?C and pH of7.2-7.4, the differences in glucose consumption (Figure 4B),DCW (Figure 4C), welan gum concentration (Figure 4D), andbroth viscosity (Figure 4E) among the three strains were notsignificant. The results revealed that the crtB deletion and irreintegration had no considerable influence on the fermentationperformance of the strains. While, when fermentation at 40?C,the four parameters above are lower than those at 30?C.However, the welan gum concentration of S. sp. NX-R canstill reach 20.26 ± 0.25 g/L compared with that of 30?C(20.85 ± 0.20 g/L), with only 2.91% decrease. Compared with S.sp. NX-3 (7.05 ± 0.15 g/L), S. sp.-1crtB (6.74 ± 0.20 g/L), thewelan gum concentration of S. sp. NX-R increased by 187.38%and 197.63%, respectively. In terms of dose, the expression ofthe global transcription factor IrrE can facilitate the tolerance ofthe welan gum-producing strain to abiotic stress and enhance itsindustrial application value.
Genome-Wide Transcriptional Analysis of IrrE-Expressing Strain in Response to Abiotic Stresses.
To understand how global transcription regulator IrrE mayconfer multiple tolerance to S. sp., we performed RNA-seq ofthree samples (S. sp. NX-R at 30?C VS S. sp. NX-3 at 30?C, andS. sp. NX-R at 40?C VS S. sp. NX-3 at 30?C) to analyze globalchanges in gene expression. Overall, the average output of eachsample was 1.34 Gb, and the clean reads, which were directlyused for subsequent analysis, accounted for more than 97% ofthe total reads.
There are 387 significantly upregulated genes and 741significantly downregulated genes in S. sp. NX-R compared withS. sp. NX-3 at 30?C (Figure 5A)。 Among them, the top 30enriched pathways of the significantly different expressed geneswere showed in the Figure 5C.The 5 most enriched pathwaysincluding atrazine degradation (ko00791), tyrosine metabolism(ko00350), nitrogen metabolism (ko00910), flagellar assembly(ko02040), glycerolipid metabolism (ko00561) etc., which areaffiliated with the xenobiotics biodegradation and metabolism,amino acid metabolism, energy metabolism, cell motility, lipidmetabolism of Kyoto Encyclopedia of Genes and Genomes(KEGG) pathway mapping. Interestingly, all Qvalues of the mostenriched pathways were greater than 0.05. These results suggestedthat expression of irre gene may have very slight effects on cellgrowth and metabolism at 30?C.
Under abiotic conditions (40?C), 946 significantly upregulatedgenes and 490 significantly downregulated genes in multiresistance strain were observed (Figure 5B)。 Compared withthe results of S. sp. NX-R at 30?C VS S. sp. NX-3 at 30?C,flagellar assembly (ko02040), bacterial chemotaxis (ko02030),two-component system (TCSs, ko02020), and cell cycle(ko04112) become the most enriched pathways (Figure 5D)。
Then, the transcription level and locations of the significantlyregulated genes involved in the pathways above are exhibitedin the Figure 5E, and as is shown, most key genes in the fourpathways are significantly upregulated. Here, TCSs, bacterialchemotaxis pathways and flagellar assembly pathways all play thefunctions of signal perception, conduction and cell motility. Thebacterial methyl-accepting chemotaxis proteins (MCPs) sensedenvironmental signals and triggered a stimulatory response,which was transmitted to the flagellar motor switch protein FliN,FliM, FliG via sensor kinase CheA and chemotaxis protein CheY.
The purine-binding chemotaxis protein CheW was also neededfor the connection of CheA and MCPs, and chemotaxis proteinmethyltransferase CheR, protein-glutamate methylesterase/glutaminase CheB, chemotaxis protein CheD were neededfor the modification of methylation and demethylation ofMCPs to adapt to the environment. Correspondingly, thetranscription levels of the flagellar biosynthesis proteinsFlhA, FlhB, FliO, FliP, FliQ, and FliR, flagellar assemblyprotein FliH, flagellar basal-body rod modification proteinFlgD, FlgE, FlgF, flagellar hook-associated protein FlgK, FlgK,and flagellar ring protein FliF, FlgI, FlgH, genes were alsosignificantly upregulated.
In the cell cycle pathway, sensor histidine kinase/responseregulator CckA, histidine phosphotransferase ChpT wasdownregulated, while cell cycle response regulator CtrAwas upregulated. Meanwhile, the chromosomal replicationinitiator protein DnaA, and the replicative DNA helicaseDnaB, the cell division protein FtsZ, FtsQ, FtsA, FtsW, theUDP-N-acetylglucosamine -N-acetylmuramyl-(pentapeptide)pyrophosphoryl-undecaprenol N-acetylglucosamine transferaseMurG, actived by DnaA and CtrA, were all upregulated, so asto propel the DNA replication S phase progression and Z-ringformation M phase progression of the cell cycle. Thus, the cellproliferation was rapidly proceed to maintain the microbialpopulation to cope with environmental challenges.
These results indicated that under the control of IrrE, thetranscription level of key genes in the pathways of signaltransduction, cell motility, cell growth and death, etc., changedsignificantly, so as to respond to the abiotic conditions andimprove the stress resistance of microorganisms.
DISCUSSION.
The high energy consumption and cost in welan gum productionprocess is a challenging issue for the current approaches. Inthis work, we selected IrrE derived from extremophiles as astress-resistant element to improve the tolerance of S. sp. against heat and weak acidity shock. We investigate key genes ofcarotenoid synthesis in S. sp. NX-3 and constructed pigmentfree cells. Afterwards, a robust welan gum production strain byintegrating IrrE into the key gene site of carotenoid synthesis wasconstructed. Finally, we investigated that IrrE plays as the globaltranscriptional regulatory factor in regulating the expressionof genes in TCSs, bacterial chemotaxis, flagellar assembly, andcell cycle pathways, which jointly promote the survival ofcells and enhance production capacity under non-physiologicalenvironmental conditions.
Many groups have attempted to manipulate transcriptionalregulation and protein post-translational modification directly orindirectly by modifying transcriptional regulator factors and heatshock proteins, to improve strain resistance, respectively. And, most results were satisfactory. Nevertheless, long experimentperiod and low success rate caused by the ambiguity of themetabolic network and the complexity of gene manipulationof the non-model strain limited the application of large-scalemetabolic engineering in S. sp. Thus, it is even more valuableto modify one or several genes to achieve multiple beneficialchanges in cell phenotype. Here, expression of the selectedglobal transcriptional regulatory factor IrrE overcame the abovementioned problems, and, it effectively reduces the demand ofcooling water, alkali binding agent, and purified alcohol in theproduction process.
Transcriptome analysis explained how strain to protect againstabiotic stresses under the control of stress-resistant element IrrE.
TCSs serve as a major stimulus-response coupling mechanismto allow organisms to sense and respond to changes in manydifferent environmental conditions (Stock et al., 2000)。 TCSscontrolled almost all bacterial physiological pathways, suchas cell-to-cell signaling, chemotaxis, sporulation, osmolarity,nutrient assimilation, cell differentiation, and virulence (Stocket al., 2000)。 Bacterial chemotaxis and flagellar assembly wereinteracted with histidine kinases (CheA) and reflex regulators(CheY) of the TCSs to stimulate the microbial chemotacticmotility to the external environment (West and Stock, 2001)。
The significant up-regulated of these genes motivated bacteria to“hasten after benefit and avoid damage.” The CckA-CtrA twocomponent signaling system was also plays an important role inthe cell cycle pathway (Biondi et al., 2006)。 Density-dependentCckA kinase phosphorylates CtrA through the single domainhistidine phosphotransferase, ChpT (Narayanan et al., 2018)。
And, the differential activity of CtrA is of paramount significancefor generating different cell fates by regulating the cell divisionprotein, modification methylase, and replicative DNA helicasedirectly or indirectly, thereby warrants the cells to replicationin large numbers to keep the population alive under nonphysiological environmental conditions (Westbye et al., 2018)。
Apart from working for cell cycle pathway, CtrA also serves asa transcription factor to drive the expression of key genes in thebacterial chemotaxis and flagellar assembly pathways. In general,under the role of the global transcriptional regulatory factorIrrE, different genes coordinated with each other to performtheir biological functions and jointly promote the survival ofcells and enhance production capacity under non-physiologicalenvironmental conditions.
CONCLUSION.
The present study aimed to solve the high energy consumptionand cost in welan gum production process. The screened globaltranscriptional regulatory factor IrrE was integrated into thekey gene site of carotenoid synthesis. Thus, a multi-resistancewelan gum-producing strain with pigment synthesis defectwas constructed. When fermented, the tolerance temperaturecan increase to 10?C without the need for pH control,and the final welan gum yield only had a slight decrease.
Transcriptome analysis showed that under the control of stressresistant element IrrE, more than 1000 genes, involved inmultiple pathways, including two-component system, bacterialchemotaxis, flagellar assembly, biofilm formation, and cellcycle, etc., exhibited changes at the transcriptional level andallowed the strain to protect against abiotic stresses. The resultswill provide a useful reference of tolerance improvement forindustrial microorganisms.
DATA AVAILABILITY STATEMENT.
The datasets presented in this study can be found in onlinerepositories. The names of the repositories and accessionnumbers can be found below: https://www.ncbi.nlm.nih.gov/,SRR11496385 https://www.ncbi.nlm.nih.gov/, SRR11496386https://www.ncbi.nlm.nih.gov/, SRR11496387.
AUTHOR CONTRIBUTIONS.
XL and SL conceived and designed the research. XL and MZperformed the research. ZX and MZ analyzed the data. XL wrotethe manuscript. SL and HX revised the manuscript. All authorscontributed to the article and approved the submitted version.
FUNDING.
This work was supported by the National Natural ScienceFoundation of China (Grant Number 21776133), the JiangsuSynergetic Innovation Center for Advanced Bio-Manufacture(Grant Number XTB1804), the Six Talent Peaks Project in JiangsuProvince (Grant Number SWYY-027), and the University ScienceFoundation of Hebei University of Economics and Business(Grant Number 2019YB07)。
SUPPLEMENTARY MATERIAL.
The Supplementary Material for this article can be foundonline at: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00674/full#supplementary-material.
REFERENCES.
Ai, H., Liu, M., Yu, P., Zhang, S., Suo, Y., Luo, P., et al. (2015)。 Improvedwelan gum production by Alcaligenes sp. ATCC31555 from pretreatedcane molasses. Carbohydr. Polym. 129, 35-43. doi: 10.1016/j.carbpol.2015.04.033.
Alper, H., Jin, Y., Moxley, J. F., and Stephanopoulos, G. (2005)。 Identifying genetargets for the metabolic engineering of lycopene biosynthesis in Escherichiacoli. Metab. Eng. 7, 155-164. doi: 10.1016/j.ymben.2004.12.003Alper, H., and Stephanopoulos, G. (2007)。 Global transcription machineryengineering: a new approach for improving cellular phenotype. Metab. Eng. 9,258-267. doi: 10.1016/j.ymben.2006.12.002.
Armstrong, G. A., Alberti, M., and Hearst, J. E. (1990)。 Conserved enzymesmediate the early reactions of carotenoid biosynthesis in nonphotosyntheticand photosynthetic prokaryotes. Proc. Natl. Acad. Sci. U.S.A. 8, 9975-9979.doi: 10.1073/pnas.87.24.9975.
Barrick, J. E., and Lenski, R. E. (2013)。 Genome dynamics during experimentalevolution. Nat. Rev. Genet. 14, 827-839. doi: 10.1038/nrg3564 .
Biondi, E. G., Reisinger, S. J., Skerker, J. M., Arif, M., Perchuk, B. S., Ryan, K. R.,et al. (2006)。 Regulation of the bacterial cell cycle by an integrated geneticcircuit. Nature 444, 899-904. doi: 10.1038/nature05321 .
Blooma, J. D., and Arnoldb, F. H. (2009)。 In the light of directed evolution:pathways of adaptive protein evolution. Proc. Natl. Acad. Sci. U.S.A. 106,9995-1000.
Chae, T. U., Choi, S. Y., Kim, J. W., Ko, Y., Lee, S. Y., and Lee, A. S. Y. (2017)。Recent advances in systems metabolic engineering tools and strategies. Curr.Opin. Biotechnol. 47, 67-82. doi: 10.1016/j.copbio.2017.06.007 .
Chen, M. (2010)。 IrrE, an Exogenous Gene from Deinococcus radiodurans,improves the growth of and ethanol production by a Zymomonas mobilis strainunder ethanol and acid stresses. J. Microbiol. Biotechnol. 20, 1156-1162. doi:10.4014/jmb.0912.12036.
Gai, Z., Wang, X., Zhang, X., Su, F., Wang, X., Tang, H., et al. (2011)。 Genomesequence of Sphingomonas elodea ATCC 31461, a highly productive industrialstrain of gellan gum. J. Bacteriol. 193, 7015-7016. doi: 10.1128/jb.06307-11 .
Gao, C., Jiang, B., Wang, Y., Liu, G., and Yang, C. (2012)。 Overexpression of a heatshock protein (ThHSP18.3) from Tamarix hispida confers stress tolerance toyeast. Mol. Biol. Rep. 39, 4889-4897. doi: 10.1007/s11033-011-1284-2.
Gao, G., Tian, B., Liu, L., Sheng, D., Shen, B., and Hua, Y. (2003)。 Expression ofDeinococcus radiodurans PprI enhances the radioresistance of Escherichia coli.DNA Repair. 2, 1419-1427. doi: 10.1016/j.dnarep.2003.08.012.
Gruber, T. M., and Gross, C. A. (2003)。 Multiple sigma subunits and thepartitioning of sacterial transcription space. Annu. Rev. Microbiol. 57, 441-466.doi: 10.1146/annurev.micro.57.030502.090913.
Jie, P., Jin, W., Zhengfu, Z., Yongliang, Y., Wei, Z., Wei, L., et al. (2009)。 IrrE,a global regulator of extreme radiation resistance in Deinococcus radiodurans,enhances salt tolerance in Escherichia coli and Brassica napus. PLoS One 2:e4422.doi: 10.1371/journal.pone.0004422.
Li, H., Xu, H., Li, S., Feng, X., Xu, H., and Ouyang, P. (2011a)。 Effects of dissolvedoxygen and shear stress on the synthesis and molecular weight of welan gumproduced from Alcaligenes sp. CGMCC2428. Process Biochem. 46, 1172-1178.doi: 10.1016/j.procbio.2011.02.007.
Li, H., Xu, H., Li, S., Xu, H., Guo, C., Ying, H., et al. (2010)。 Strain improvementand metabolic flux modeling of wild-type and mutant Alcaligenes sp. NX-3for synthesis of exopolysaccharide welan gum. Biotechnol. Bioprocess Eng. 15,777-784. doi: 10.1007/s12257-010-0021-3.
Li, H., Xu, H., Xu, H., Li, S., Ying, H., and Ouyang P. (2011b)。 Enhanced welan gumproduction using a two-stage agitation speed control strategy in Alcaligenessp CGMCC2428. Bioprocess Biosyst. Eng. 1, 95-102. doi: 10.1007/s00449-010-0450-6.
Lin, Z., Zhang, Y., and Wang, J. (2013)。 Engineering of transcriptional regulatorsenhances microbial stress tolerance. Biotechnol. Adv. 31, 986-991. doi: 10.1016/j.biotechadv.2013.02.010.
Lindquist, S. (1992)。 Heat-shock proteins and stress tolerance in microorganisms.Curr. Opin. Genet. Dev. 2, 748-755. doi: 10.1016/s0959-437x(05)80135-2.
Liu, X., Zhu, P., Jiang, R., Wu, L., Feng, X., Li, S., et al. (2017)。 Enhancement ofwelan gum production in Sphingomonas sp. HT-1 via heterologous expressionof Vitreoscilla hemoglobin gene. Carbohydr. Polym. 156, 135-142. doi: 10.1016/j.carbpol.2016.08.081.
Marc, J., Grousseau, E., Lombard, E., Sinskey, A. J., Gorret, N., and Guillouet, S. E.(2017)。 Over expression of GroESL in Cupriavidus necator for heterotrophicand autotrophic isopropanol production. Metab. Eng. 42, 74-84. doi: 10.1016/j.ymben.2017.05.007.
Mukhopadhyay, A. (2015)。 Tolerance engineering in bacteria for the production ofadvanced biofuels and chemicals. Trends Microbiol. 23, 498-508. doi: 10.1016/j.tim.2015.04.008.
Narayanan, S., Kumar, L., and Radhakrishnan, S. K. (2018)。 Sensory domainof the cell cycle kinase CckA regulates the differential DNA binding of themaster regulator CtrA in Caulobacter crescentus. Biochem. Biophys. Aeta 1861,952-961. doi: 10.1016/j.bbagrm.2018.08.006.
Preejith, V., Prakash, B., and Bernstein, P. S. (1996)。 Microbial carotenoids. Adv.Biochem. Eng. Biotechnol. 898, 119-178. doi: 10.1007/bfb0102327.
Santos, C. N. S., and Stephanopoulos, G. (2008)。 Combinatorial engineering ofmicrobes for optimizing cellular phenotype. Curr. Opin. Chem. Biol. 2, 168-176. doi: 10.1016/j.cbpa.2008.01.017.
Stock, A. M., Robinson, V. L., and Goudreau, P. N. (2000)。 Two-Component SignalTransduction. Annu. Rev. Biochem. 69, 183-215.
Suo, Y., Luo, S., Zhang, Y., Liao, Z., and Wang, J. (2017)。 Enhanced butyricacid tolerance and production by Class I heat shock protein-overproducingClostridium tyrobutyricum ATCC 25755. J. Indus. Microbiol. Biotechnol. 44,1145-1156. doi: 10.1007/s10295-017-1939-7.
Tanaka, K., Ishii, Y., Ogawa, J., and Shima, J. (2012)。 Enhancement of acetic acidtolerance in Saccharomyces cerevisiae by overexpression of the HAA1 gene,encoding a transcriptional activator. Appl. Environ. Microbiol. 78, 8161-8163.doi: 10.1128/aem.02356-12.
Tang, H., Wang, X., Bowers, J. E., Ming, R., and Alam, M. (2008)。 Unravelingancient hexaploidy through multiply-aligned angiosperm gene maps. GenomeRes. 18, 1944-1954. doi: 10.1101/gr.080978.108.
Wang, X., Tao, F., Gai, Z., Tang, H., and Xu, P. (2012)。 Genome sequence of thewelan gum-producing strain Sphingomonas sp. ATCC 31555. J. Bacteriol. 194,5989-5990. doi: 10.1128/jb.01486-12.
West, A. H., and Stock, A. M. (2001)。 Histidine kinases and response regulatorproteins in two-component signaling systems. Trends Biochem. Sci. 26, 369-376. doi: 10.1016/s0968-0004(01)01852-7.
Westbye, A. B., Kater, L., Wiesmann, C., Ding, H., Yip, C. K., and Beatty, J. T.(2018)。 The protease ClpXP and the PAS domain protein DivL regulate CtrAand gene transfer agent production in Rhodobacter capsulatus. Appl. Environ.Microbiol. 84, 275-301.
Wu, X., Li, O., Chen, Y., Zhu, L., Qian, C., Teng, Y., et al. (2011)。 A carotenoid-freemutant strain of Sphingomonas paucimobilis ATCC 31461 for the commercialproduction of gellan. Carbohydr. Polym. 84, 1201-1207. doi: 10.1016/j.carbpol.2011.01.018.
Xie, C., Mao, X., Huang, J., Ding, Y., Wu, J., Dong, S., et al. (2011)。 KOBAS 2.0: aweb server for annotation and identification of enriched pathways and diseases.Nucleic Acids Res. 39, 316-322.
Yu, A., Li, P., Tang, T., Wang, J., Chen, Y., and Liu, L. (2015)。 Roles of Hsp70s instress responses of microorganisms, plants, and animals. Biomed. Res. Int. 2015,1-8. doi: 10.1155/2015/510319.
Zhang, W., Chen, Z., Wu, M., Shi, Z., Zhu, F., Li, G., et al. (2016)。 Improvedproduction of carotenoid-free welan gum in a genetic-engineered Alcaligenessp. ATCC31555. Biotechnol. Lett. 38, 991-997. doi: 10.1007/s10529-016-2068-5Zhu, L., Xia, S., Wei, L., Li, H., Yuan, Z., and Tang, Y. (2016)。 Enhancing succinicacid biosynthesis in Escherichia coli by engineering its global transcriptionfactor, catabolite repressor/activator (Cra)。 Sci. Rep. 6:sre36526.
Zhu, P., Chen, X., Li, S., Xu, H., Dong, S., Xu, Z., et al. (2014a)。 Screening andcharacterization of Sphingomonas sp. mutant for welan gum biosynthesis atan elevated temperature. Bioprocess Biosyst. Eng. 37, 1849-1858. doi: 10.1007/s00449-014-1159-8.
Zhu, P., Dong, S., Li, S., Xu, X., and Xu, H. (2014b)。 Improvement of welan gumbiosynthesis and transcriptional analysis of the genes responding to enhancedoxygen transfer by oxygen vectors in Sphingomonas sp. Biochem. Eng. J. 90,264-271. doi: 10.1016/j.bej.2014.06.011.
Zingaro, K. A., and Terry Papoutsakis, E. (2013)。 GroESL overexpression impartsEscherichia coli tolerance to i-, n-, and 2-butanol, 1,2,4-butanetriol and ethanolwith complex and unpredictable patterns. Metab. Eng. 15, 196-205. doi: 10.1016/j.ymben.2012.07.009.
Conflict of Interest: The authors declare that the research was conducted in theabsence of any commercial or financial relationships that could be construed as apotential conflict of interest.
Copyright ? 2020 Liu, Zhao, Xu, Xu and Li. This is an open-access article distributedunder the terms of the Creative Commons Attribution License (CC BY)。 The use,distribution or reproduction in other forums is permitted, provided the originalauthor(s) and the copyright owner(s) are credited and that the original publicationin this journal is cited, in accordance with accepted academic practice. No use,distribution or reproduction is permitted which does not comply with these terms.
您好!根据您的论文需求,我们整理了一篇题为 《 足球运动员比赛前、中、后所需的营养探究 》 的足球与营养相关的论文文献供您参考,论文部分内容如下,文末有阅读全文链接。 ( 注:如果本篇文章无法满足您的需求,您可以再次使用文献求助,注明所需文献标...
Landscape architecture projects can vary from small-scale house gardens to undertakings complementing regional and urban planning. From the private property of a single family to the most crucial public spaces in the cit...
文章孔子的生态伦理思想理念探究原文出处: 吴晓红,袁媛。孔子生态伦理思想及其当代价值探究[J].蚌埠学院学报,2019,8(03):90-93. 注:本回复末尾将附上该文全文链接,有需要的朋友可以自行点击阅读! 该文主要对回望儒家生态伦理学源流,以孔子生态伦理...
《安徽农村金融》杂志最近1期更新于2011年12月,后续时间已经停更,下面根据您的需求,为您找到一篇期刊当中的论文《欠发达地区农村信用社经营中存在的问题及创新》,全文如下: 欠发达地区农村信用社经营中存在的问题及创新 作者:谢云 单位:湖北省荆州市...
音色是演员刻画人物的必要条件, 当演员拿到角色进行研究与创造, 合理地修饰语言状态与音色能够更加丰富演员塑造的人物形象。演员在自己的演艺生涯中会遇到各种各样不同类型的角色, 通过修饰加上自己的舞台经验让自己在塑造人物时候能更加真实, 更好地提...
你好!根据你的需求,学术堂将网站原文章进行了修改完善,并提供该文全文免费下载,详情点击查看 C/S结构下典当行业务管理系统的设计研究 同时我们还找到一篇典当信息管理系统开发相关硕士论文,以下内容节选自硕士论文《基于B/S模式的典当信息管理系统设计...
你好,根据你的需求,没有找到完全满足机场运行效率大数据可视化分析的现成论文,建议你参考机场运行效率分析和大数据可视化分析案例方向的相关文献,然后将两者结合撰写你的论文。 以下是4篇关于机场运行效率分析和大数据可视化分析相关的参考文献,供您参...
你好!根据你的需要,学术堂收集整理了6篇关于红色旅游资源开发的研究文献,其中包含国内外研究综述、当前开发问题与对策等,以下是相关文献: 下面是1篇关于红色旅游发展的研究文献综述 原文出处:谢晓敏.红色旅游发展的文献综述[J].区域治理,2019(35):...
你好,根据你提出的需求,学术堂精选2016年到2020年35篇财务报表分析相关的外文文献供您参考,如需更多外文文献,可联系我们,或提交求助获取. 财务报表分析外文文献一: [1]Ken B. Cyree,Travis R. Davidson,John D. Stowe. Forming appropriate peer group...
任何一种风格的流行,都是在更好地表达高品质生活方式,以及彰显居家态度。关于轻奢风格,“轻”是低调舒适,有品质;“奢”是追求奢华,有品味。...