求助主题我需要一篇doi.org/10.1016/j.urology.2020.12.022的文章
需求说明
求助时间2021-01-29 11:40
您好!根据你的需求,学术堂查找到doi.org/10.1016/j.urology.2020.12.022的外文文献,其标题为《The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial》,现整理出其部分内容供您确认,如需查看全文请到文章末尾处免费下载。
The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial
Bilal Chughtai*, Dean Elterman*, Neal Shore, Marc Gittleman, Jay Motola, Sheldon Pike,Craig Hermann, William Terrens, Alfred Kohan, Ricardo R. Gonzalez, Aaron Katz,Jeffery Schiff, Evan Goldfischer, Ivan Grunberger, Le Mai Tu, Mark N. Alshak, and Jed Kaminetzky
论文摘要:
OBJECTIVE:To report the results of a multicenter, randomized, controlled trial with a temporarily implanted nitinol device (iTind; Medi-Tate Ltd, Hadera, Israel) compared to sham for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia.
MATERIALS AND METHODS:Men 50 years or older were randomized 2:1 between iTind and sham procedure arms. A self-expanding, temporary nitinol device was placed for 5-7 days and an 18F Foley catheter was inserted and removed for the iTind and sham group, respectively. Patients were assessed at base-line, 1.5, 3, and 12 months postoperatively using the IPSS, peak urinary ow rate, residual urine,quality of life, and the International Index of Erectile Function. Unblinding occurred at 3 months.
RESULTS:A total of 175 men (mean age 61.1 § 6.5) participated (118 iTind vs 57 sham)。 A total of 78.6% of patients in the iTind arm showed a reduction of ≥3 points in IPSS, vs 60% of patients in the control arm at 3 months. At 12 months, the iTind group reported a 9.25 decrease in IPSS (P< .0001), a 3.52ml/s increase in peak urinary ow rate (P < .0001) and a 1.9-point reduction in quality of life (P < .0001)。 Adverse events were typically mild and transient, most Clavien-Dindo grade I or II, in 38.1% of patients in the iTind arm and 17.5% in the control arm. No de novo ejaculatory or erectile dysfunction occurred.
CONCLUSION:Treatment with the second-generation iTind provided rapid and sustained improvement in lower uri-nary tract symptoms for the study period while preserving sexual function. UROLOGY 00: 17, 2020. 2020 Elsevier Inc.
论文正文:
Benign prostatic hyperplasia (BPH) is often associ-ated with bothersome lower urinary tract symp-toms (LUTS) affecting 50%-75% in men over 50,and reach up to 80% in men aged 70 years and older with adherence rates to pharmacology as low as 30% after one year due to unmet due to unmet patient expectations or bothersome side effects.1-5 Treatment-induced sexual dysfunction is a key concern when considering pharma-ceutical therapy.3,6,7
Transurethral resection of the prostate (TURP), con-sidered the “gold-standard” in surgical therapy for LUTS secondary to BPH, provides signicant and durable relief of symptoms. However, it incurs the risk of signicant postprocedural morbidity and long-term complications, including urinary incontinence (3%), strictures (7%),erectile dysfunction (10%), and loss of ejaculation (65%),and 20%-50% persistent LUTS.8,9 Novel laser-based ablative modalities provide effective relief of BPH-related symptoms with a similar rate of complications as TURP.8,10
Among effective, minimally invasive alternatives for LUTS secondary to BPH treatment such as the prostatic urethral lift (PUL, UroLift System, Extract, CA) 11 and convective water vapor treatment (Rezum System,NxThera, Maple Grove, MN), the second-generation temporarily implanted nitinol device (iTind; Medi-Tate Ltd, Hadera, Israel)11 was given de novo authorization by the Food and Drug Administration (FDA) in February 2020. Two single-arm studies have demonstrated that iTind treatment provides rapid and effective LUTS relief that is durable to 3 years, with low rates of adverse events (AEs) and preservation of sexual function.12,13 This study compares iTind to sham in the reduction of LUTS sec-ondary to BPH.
MATERIALS AND METHODS
Study Protocol and Objectives
This prospective, randomized, controlled, single-blinded study of the second-generation iTind procedure was conducted at 16 sites in the US and Canada in men with symptomatic BPH. The FDA and Health Canada approved the study, as did institutional review boards at each of the enrolling sites (Clinicaltrials.gov:NCT02506465)。 Written informed consent was obtained from all participants.
Subjects eligible and enrolled for the study included: men ≥ 50 years, IPSS (International Prostate Symptoms Score) of ≥10,peak urinary ow rate (PFR) of ≤12 mL/sec with a 125 mL voided volume, prostate volume between 25 and 75 cc, and nor-mal urinalysis, complete blood count, and biochemistry.
Excluded patients had a postvoid residual volume (PVR) >250 mL, obstructive median lobe (OML), prostate specic antigen (PSA) >10 ng/mL or free PSA < 25%, without a subse-quent negative prostate biopsy, previous prostate surgery, pros-tate or bladder cancer, neurogenic bladder and/or sphincter abnormalities, or confounding bladder pathologies based on medical history, recent cystolithiasis or hematuria, active UTI,compromised renal function, severe respiratory disorders, known immunosuppression, active antithrombotic or antiplatelet treat-ment, cardiac disease, including arrhythmias and uncontrolled diabetes mellitus. Ultrasound was carried out pre-operatively to evaluate for OML and IPP was measured. Cystoscopy was not mandatory during screening, but cystoscopy was used during placement of the device and an intra-operative exclusion crite-rion of OML existed.
Baseline medical history, BPH-related medications, uroow-metry, IPSS, PVR, and completion of questionnaires regarding quality of life (QoL), erectile, and ejaculatory function was col-lected before the procedure. All patients on BPH-related medi-cations started a wash-out period prior to implantation: 1 month for alpha-blockers and 6 months for 5-alpha-reductase inhibitors. Medication nave patients seeking treatment refused medication in preference for a minimally invasive sur-gical technique.
iTind Procedure
The iTind implantation has been described in previous stud-ies.12,13 Briey, the iTind device is comprised of three elongated,intertwined nitinol struts at the 12, 5, and 7 o'clock positions, an anti-migration anchoring leaet at 6 o'clock, and a polyester retrieval suture for easy device removal.
The device is implanted for 5-7 days, during which it expands and exerts radial force, creating deep ischemic incisions, and a remodeling on the prostate tissue at the bladder neck and ante-rior prostatic fossa. The iTind is deployed under direct visualiza-tion in an ambulatory procedure using a rigid cystoscopy. The device is removed through either a rigid cystoscope or an open-ended 22F Foley catheter with topical anaesthesia. Both implan-tation and removal can be done under local, IV, or general anaesthesia at the discretion of the performing physician. Cathe-terisation is not required following either implantation or removal.
Sham Procedure
The sham control was the insertion and removal of an 18F sili-con Foley catheter in order to simulate both the implantation and retrieval procedures. Throughout the procedure, the surgeon gave verbal description as if deploying the iTind device, after which the catheter was removed. A similar protocol was fol-lowed for the removal. Although the iTind device is deployed through a rigid cystoscope, a Foley catheter was used to minimize the risk of procedure-related morbidity. Subjects in both the device and control groups were draped to prevent them from see-ing the treating physician and the device.
Statistical Methodology
Subjects were randomized in 2:1 ratio to either iTind or control groups using permuted blocks stratied by center by using a cen-tral electronic data program. Analysis suggested that for an expected response rate of 75% in iTind and 51% in sham (24% difference), using a 5% 2-tailed Fisher exact test, a total of 180 subjects randomized to either iTind or sham will provide at least 85% power to meet the study primary endpoint.
The primary endpoint compared the percentage of patients achieving a reduction of at least 3 points in IPSS at 3 months,between iTind and control groups, in accordance with the FDA guideline for BPH treatments, published in 2010, the most recent guidance available at the time of the study. Similar to other randomized controlled trials, unblinding of the sham arm occurred at 3 months.14,15
The ITT analysis used logistic regres-sion with baseline IPSS, diagnosis of BPH at baseline, prostate volume, and PSA as covariates. Missing IPSS were imputed using multiple imputations under the “missing at random” assumption. The imputed model included treatment group,country/geographical region pre-study diagnosis of BPH, baseline prostate volume, and PSA. Missing prostate volume and PSA were imputed for 2 patients based on baseline values of PFR, PVR, IPSS, IIEF, SHIM, and BPH. Patients who discontinued the study due to AEs or initiation of alternative treatments were considered treatment failures and were imputed as a worst-case imputation, which is a baseline value for all missing evaluations.The responder analysis was also carried out at 12 months to eval-uate durability of effect.
All secondary endpoints were analyzed descriptively in the per protocol analysis set of eligible patients.Statistical analysis was done using SAS 9.4 (SAS Institute Inc., Cary, NC)。 Statistical signicance was accepted at P-value < .05.
RESULTS
Procedure
A total 185 men (mean age 61.1 § 6.5 years, mean BMI 28.8 §5.7 kg/m2) were randomized 2:1 and assigned to either treatment with iTind (n = 128) or sham control (n = 57) in 16 centers (14 in the United States, 2 in Canada) between July 2015 and Octo-ber 2018 (Table 1)。 Baseline demographics were similar among randomized groups, except for the Charlson Comorbidity Index,with iTind having a higher score (2.52 v. 1.26, P< .001)(Table 1)。 All sites had no prior experience with iTind. Notably,213 screen failure events occurred, the majority of which were due to not meeting the inclusion criteria. Three were due to patients refusing a wash-out of 5-alpha-reductase inhibitors (6mos)。 10 men did not undergo the iTind procedure, resulting in a nal cohort of 118. Reasons for loss of follow-up can be found in Figure 1. Medication nave patients seeking treatment refused medication in preference for a minimally invasive surgi-cal technique. No cases were excluded intra-operatively due to OML.
Table 1. Baseline Demographics
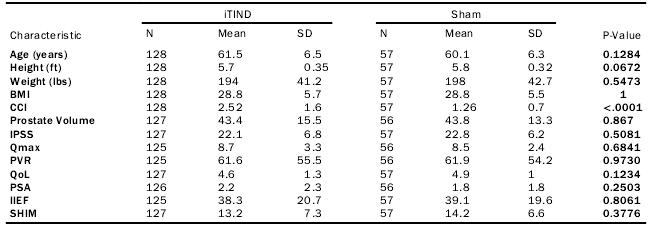
Efficacy
The primary endpoint of improvement of IPSS ≥ 3 determining the effectiveness of the iTind treatment was achieved at 3 months in 78.6% of iTind patients compared to 60% of patients in the control arm, a difference of 18.6% (P= .029)。 The responder analysis of improvement of IPSS ≥ 7 showed that iTind still had a responder's rate of 72.6% vs 50% in the sham arm (P = .048)。 A durable responder rate in 79% of patients was demonstrated out to 12 months (P = .009)。 Of note, 97% of patients that responded to treatment at 3 months remained res-ponders at 12 months.
At the time of unblinding at 3 months, in the ITT patient population, iTind improved IPSS by -9.0 § 8.5 (22.1-13.0) while the sham arm improved -6.6 § 9.5 (22.8-15.8) (P = .063)。
iTind and sham also demonstrated an improvement in QoL in the ITT patient population, with a reduction from 4.6 § 1.3 at baseline to 2.7 § 1.8 at 3 months, vs 4.9 § 1.0-3.4 § 2.0 in the sham arm, respectively (P = .264)。 Similarly, PFR improved from 8.7 § 3.3 mL/s to 13.1 § 7.1mL/s in the iTind arm vs 8.5 § 2.4 mL/s-11.4 § 5.3mL/s in the sham arm at 3 months (P = .230)。 Additionally, PVR improved from 60.78 § 56.35 mL to 59.44 § 56.43 mL in the iTind arm vs 61.9 § 54.2 mL-66.9 § 65.1 mL in the sham arm at 3 months (P = .781)。 Sexual function according to the IIEF and SHIM questionnaires remained unchanged in both groups.
Secondary endpoints in the iTind arm showed a signicant reduction in IPSS urinary symptoms at 12 months from 22.64 § 6.8 at baseline to 12.69 § 6.35; a reduction of -9.25 § 6.49 points in the per protocol population (P < .0001) (Table 2)。
Of note, patients that were severely symptomatic at baseline (IPSS of 20-35) reported a similar level of improvement at 12 months to those that were moderately symptomatic (IPSS of 8-19), with a reduction of 41.5% vs 39%, respectively.QoL score was reduced from 4.51 § 1.24 to 2.45 § 1.79 (-1.9 § 1.74) at 12 months (P < .0001), and PFR also increased from 8.42 § 2.09 mL/s to 11.93 § 4.89 mL/s (3.52 § 5.24 mL/s) (P < .0001) (Table 2)。
PVR only signicantly decreased at the 1.5-month follow-up (65.08 § 60.66-49.90 § 55.82, P = .0244) but did not decrease with any clinically signicant change from baseline at 3 months and 12 months among men who voided at least 125cc (Table 2)。
Six men (4.7%) had an alternative BPH surgery during the 12-month follow-up due to deterioration of symptoms. iTind did not complicate any of the alternative surgeries. An additional 6 men (4.7%) required medication for LUTS secondary to BPH (Fig. 1)。
Safety
In the iTind group, a total of 5 procedure- and/or device-related SAEs were observed in 3 patients, including urinary retention (n = 2), UTI (n = 2), and sepsis (n = 1) (Table 3)。 Urinary reten-tion, UTI, and sepsis did not occur in the sham arm. Only 3 SAEs from 2 patients during the post retrieval phase were found to be possibly related to the device. One patient died from an unrelated pancreatic cancer complication, as adjudicated by clinical events committee and data monitoring committee.
iTind had more overall AEs as compared to the sham group within the rst 30 days (38.1% vs 17.5%)。 68% of AEs occurred within 7 days of treatment (while the device was in the body)。
Most were mild, anticipated, and all but 2 resolved within 1- 4 weeks. Dysuria occurred in 22.9% of men and hematuria in 13.6% in the iTind arm compared to 8.8% and 0% in the sham arm within the rst 30 days. None of the 118 subjects experi-enced de novo erectile or ejaculatory dysfunction (Table 2)。SHIM for patients at baseline was 12.92 § 7.49 with no change at 1.5, 3, or 12 months (P= .8165, P = .7078, and P = .3155, respectively) and IIEF at baseline for patients was 36.86 § 20.04 with no change at 1.5 and 3 months (P = .0738 and P = .0523) with an increase in score of 4.51 § 18.10 at 12 months (P = .0101) (Table 2)。 All procedures were performed without serious perioperative AEs.
The iTind implantation procedure was well tolerated, with a mean post-procedural VAS pain score of 4.2 (SD: 3.1) vs 1.0 (2.2) for the sham arm. Removal of iTind had a mean VSAS score of 3.3 (3) vs 2 (2.1) in the sham arm. iTind implantation procedures were performed under IV sedation (n = 77, 66.1%), local anesthetic (n = 32, 27.1%), or general anesthesia (n = 8, 6.8%)。 iTind removal was performed under IV sedation (n = 71,60.2%), local anesthetic (n = 29, 24.6%), or general anesthesia (n = 3, 2.5%)。 Sham procedure was performed under IV sedation (49.2%), local anesthetic (49.2%), or general anesthesia (1.8%)。
All patients were discharged day of procedure. iTind patients reported a return-to-preoperative-activity level of 5.2 § 17.0 days after device retrieval, compared to 3.5 § 4.4 days for control. None of the subjects in the iTind arm underwent rou-tine postoperative catheterization, with only 7 (5.9%) of patients in the iTind arm experiencing an episode of urinary retention.
DISCUSSION
The results of this randomized, controlled, single-blinded, double-arm prospective study on the iTind device demon-strate improvements in IPSS of -9.0 § 8.5 points (40.1%) at 3 months. These results substantiate previous prospec-tive studies on iTind that showed reductions in IPSS at 3 months of 11 points and 12.6 points, respectively.12,13
This improvement in IPSS is similar to other minimally invasive devices. PUL demonstrated a reduction in IPSS of 11.1 points at 3 months, and Rezum demonstrated a similar reduction of 11.3 points in the same follow-up period.14,15
Additionally, iTind showed a mean improve-ment in PFR of 4.4 mL/s at 3 months, which is also consis-tent with PUL and Rezum results.14,15
Treatment with iTind demonstrated to be durable for 12 months. IPSS improvement was maintained with a mean reduction of -9.25 § 6.49 points from baseline, and PFR, with an average increase of 3.52 § 5.24 mL/s from baseline. Men with severe or moderate symptoms at base-line had similar rates of improvement at 12 months. Only 4.7% of patients underwent another surgical intervention for BPH during the follow-up period. Previous studies have demonstrated similar results. In the rst study, no patients required surgical intervention for BPH after 3 years, and the second study presented a total, accumu-lated re-intervention rate of 8.6% at 2 years of follow-up following iTind placement.12,13
Importantly, the iTind procedure can be conducted with the patient under sedation or local anesthesia in an ambulatory or ofce setting, with almost 50% of patients being treated in the clinic outpatient setting. Our study also supports iTind placement is a catheter-free procedure,with only 7 patients experiencing self-resolving urinary retention. Most patients returned to their pre-operative activity level within 5 days from the retrieval of the device.
Preservation of ejaculatory function is of major impor-tance to men when pursuing treatments for LUTS second-ary to BPH.17
TURP is associated with rates of retrograde ejaculation of 38.2%-89.0% and impotence rates of 13.0%-14.0%, while laser prostatectomy has retrograde ejaculation rates of 50%-76.6% and impotence rates of 5.2%-7.9%.18
Both surgical and pharmacologic sexual side effects contribute to the undertreatment of men with BPH.3,6,7
One major advantage of the iTind procedure is the preservation of sexual function. No iTind subjects experienced de novo erectile dysfunction or retrograde ejaculation. This is similar to a recent prospective study showing sexual function was preserved in all iTind sub-jects at 6 months of follow-up.19
AEs were limited to mild events at a low rate. Medica-tion-use was low, with only one patient complaining of pain. Our low-rate of SAEs is important, given that TURP can lead to as much as 9% of cases (blood transfu-sion, sepsis, and deep venous thromboembolism,etc.)。20,21
Importantly, our observed procedure-related AEs were also less than that with other transurethra procedures, with 33.1% of patients experiencing an AE, vs 38% of Rezum patients and 80% of PUL patients. Less SAEs (dysuria, hematuria, pollakiuria, micturition urgency) were comparable to other minimally invasive endourological therapies as well as standard cystos-copy.14,15
The most common AEs with iTind were dysuria (22.9%) and hematuria (13.6%), PUL had dysuria and hematuria rates of 34.3% and 25.7% while Rezum had dysuria and hematuria rates of 16.9% and 11.8%, respec-tively. The absence of the need for postoperative catheter-ization also results in a lower rate of UTIs with an incidence of 1.7% vs 2.9% with PUL and 3.7% with Rezum.14,15
As shown in earlier trials, since no device is left in the body long-term, there is nothing to complicate future MRI-guided prostate biopsy, and no risk of delayed AEs out to 12 months.12,13
Our study has several limitations. First, we had a loss of follow-up between the baseline groups at the 3-month visit of 29% of patients in the iTind arm, and 30% of patients in the sham arm, which may have skewed the results. While our lost to follow-up was high, there was a matched dropout rate between the iTind and sham arm, showing that this was likely not a procedure-related drop-off. Missing values for various endpoints were lled in using the “missing at ran-dom” assumption to help overcome this. Moreover, because our study included specic inclusion criteria in regards to age, IPSS, PFR, and prostate volume, our results are not gen-eralizable to all men with LUTS secondary to BPH. Further-more, our study did include a powerful placebo effect that resulted in non-statistically signicant improvement in iTind versus the sham arm at the time of unblinding at 3 months.This can partly be explained by the brain's response to treat-ment, including a sham procedure.22
Moreover, a meta-anal-ysis found signicant improvements in AUA-SS and Qmax at 3 months in the sham arm of randomized controlled trials in BPH trials.23 AUA-SS improved an average of 27%, simi-lar to our improvement in IPSS of 28.9% in our sham arm.While our sham effect is large, this improvement is similar to PUL's sham arm improvement of 24.2% and Rezum's sham arm improvement of 20%.14,16 Strengths of our study include that it was randomized, blinded, and conducted at
16 sites comprised of a variety of types of care facilities, from ofces to university hospitals who all had no previous experi-ence with the procedure.
CONCLUSION
iTind provides a safe, rapid, and sustained improvement in LUTS to 12 months secondary to BPH in prostate vol-umes of 25-75cc. This minimally invasive FDA-approved procedure is effective and well tolerated for LUTS treat-ment, while preserving both ejaculation and erectile func-tion, and offers patients an attractive alternative for relief of symptomatic BPH.
全文图表和参考文献省略。