Abstract: OBJECTIVE: To investigate immunomodulatory effects of Astragalus polysaccharides(APS) on the co-culture of peripheral blood mononuclear cells(PBMCs) with He La cervical cancer cell line.METHODS: To assess the proliferation of PBMCs,carboxyfluorescein succinimidyl ester(CFSE)-labeled PBMCs were co-cultured with He La cells and treated with different concentrations of APS. Supernatants of cell culture were collected for cytokines assay via enzyme-linked immunosorbent assay(ELISA). The impact of APS on the proliferation of PBMCs, induction of regulatory T cells(Tregs), and myeloid-derived suppressor cells(MDSCs) was carried out by flow cytometry.RESULTS: It was observed that APS could increase the proliferation of PBMCs co-cultured with He La cells(P < 0.05). However, APS had no significant effects on the induction of Tregs and MDSCs in the co-culture assay(P > 0.05). Furthermore, ELISA results demonstrated that APS could decrease IL-10 and TGF-β concentration(P < 0.05).CONCLUSION: The above-mentioned characteristics showed that APS might be able to modulate immune responses and improve anti-tumor effects through increasing the proliferation of PBMCs and decreasing inhibitory cytokines secretion as critical mediators of immune suppression in the tumor microenvironment.
Keyword: Astragalus; polysaccharides; uterine cervical neoplasms; He La cells; coculture techniques; cytokines; T-lymphocytes,regulatory;
Cervical cancer (CC) is known as the fourth frequent cancer type in women with an approximately estimated 570 000 new cases in 2018.[1,2]However,traditional therapies,such as chemotherapy,radiotherapy,and surgical resections have mostly been applied to improve clinical symptoms of cancer patients and tumor remissions.Given the adverse complications of such therapies,immunomodulatory components extracted from traditional medicinal plants are recently being investigated as effective therapeutic approaches for modulation of suppressive immune responses in the tumor microenvironment (TME).[3]TME has been accepted as an aiding factor to cancer progression.In this regard,the TME components include fibroblasts,blood and lymphatic vascular networks,immune and inflammatory cells,as well as adipose-related cells.Accordingly,regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) are two types of immune suppressor cells that may play leading roles in TME that were also studied in the present study.[4,5,6]) Radix Astragali Mongolici (Huangqi in chinese) is known as a medicinal herb used in Traditional Chinese Medicine for centuries.[7]The main constituents of this herb are polysaccharides,saponins,flavonoids,amino acids including gamma-aminobutyric acid (GABA) and L-canavanine,and trace elements.There are also 14 polysaccharides in the dried root of Astragalus membranaceus (Fisch.) whose13 types possessβ-D-(1→3)-galactan moieties split withβ-D-(1→6)-galactooligosaccharide side-chains.[8]As the main active extract from astragalus membranaceus,APS has been associated with a variety of proprieties like immune-stimulant,anti-inflammatory,anti-oxidative,anti-diabetic,hepato-protective,anti-viral,and anti-cancer activities[9]as well as several biological effects based on the regulation of cellular and humoral immunity.[10,11]Additionally,numerous studies have reported that APS has strong immunomodulatory effects in vitro/in vivo that may be involved in inhibiting tumor growth,facilitating cytokine secretion,and promoting immune organ indices in Ehrlich ascites carcinoma (EAC)-bearing mice.[6,12]
However,the immunomodulatory effects of APS on PBMCs co-cultured with CC cells have not been so far elucidated.Herein,the purpose of in vitro co-culture of PBMCs and He La cells was to investigate the immunomodulatory effects of APS on the proliferation of PBMCs,induction of Treg cells,and MDSCs,as well as cytokine secretion by PBMCs co-cultured with He La cells.

Reagents
APS was purchased from NOW Foods (Bloomingdale,IL,USA).It was subsequently dissolved in normal saline to develop a final stock solution of 5 mg/m L.The given solution was subsequently filtered utilizing a0.22?pore filter.Further dilution was also prepared in the complete Roswell Park Memorial Institute Medium1640 (RPMI 1640) medium.RPMI 1640 medium,penicillin/streptomycin,fetal bovine serum (FBS),and trypsin were purchased from Gibco (Amarillo,Texas,USA).Additionally,3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent was obtained from Sigma-Aldrich,Germany.The interferon (IFN)-γ,TGF-β,IL-10,IL-6,and Vascular endothelial growth factor (VEGF) levels were determined using a commercial ELISA kit (BD Biosciences,San Diego,CA,USA).CFSE (Waltham,Massachusetts,USA) was used for proliferation assay and then stored at-20℃in dark bottles with a final concentration of5 m M.The frequency of MDSCs and Treg cells was determined on a fluorescence-activated cell sorter (FACS)caliber (BD Biosciences) with the following markers:FITC-labeled anti-human CD4 (clone RPA-T4),APC-labeled CD25 (M-A251),PE-labeled CD127(HIL-7R-M21),PE-labeled HLA-DR (G46-6),APC-CY7-labelled CD11b (ICRF44),and APC-labeled anti-CD33 (WM53)(all from BD Biosciences)based on manufacturer's instructions following isotype-matched controls.
MTT cytotoxicity assay
The effect of APS extract on the viability of PBMCs and He La cells was determined using MTT assay.PBMCs and He La cells (15×10[4]and 1×10[4]cells/well,respectively) were cultured in separate wells using96-well plates.These cells were treated with various APS concentrations (1000,500,250,125,60,30,15?g/m L) and incubated for 24,48,72,and 96 h at37℃and 5%CO2.The cells were also cultured alone(a negative control) and treated with 5?g/m L of Polyhydroxyalkanoates (PHA),as a positive control.The experiments were performed in triplicate.In this respect,following each different time group,20?L of an MTT solution at 5 mg/m L was added into each well and thenceforward incubated for 4 h in darkness at 37℃and 5%CO2.Afterward,the medium along with the MTT solution was removed from every well and100?L DMSO (Fisher Scientific,LE,UK) was then added into each one.Ultimately,the absorbance was calculated at 570 nm wavelength through ELISA Reader (Labtech LT-4000 Microplate Reader) and it was then directly correlated with viable cultured cells.Dividing the absorbance of treated cells by that of the control group in each experiment,the relative percentage of cell survival was also calculated.The extracts of APS that demonstrated no toxicity on PBMCs and He La cells were further tested for proliferative effects of PBMCs by using CFSE dye dilution assay.
Proliferation Assay of PBMCs
The proliferative effects of the extracts of APS on PBMCs were performed using CFSE staining.Human PBMCs were separated by gradient centrifugation using Ficoll (Lymphodex,Germany) at 400×g for 20 min at20℃.The PBMCs were then collected and washed with phosphate buffered saline (PBS).Briefly,PBMCs(10×10[6]cells/well) were labeled with 5?M of CFSE for 5 min at 37℃in darkness.The reaction was completed by adding PBS supplemented with 5%FBS.The CFSE-labeled PBMCs were then plated in 24-well plates (5×10[5]cells/well) and cultured for 4 d with or without APS concentrations (1000,500,250,125,60,30,15?g/m L).The culture supernatants were collected and immediately frozen at-80℃for subsequent cytokine assays.
In vitro interactions of PBMCs and He La cells
He La CC cell line (human cervical adenocarcinoma,ATCC#HTB-26) was purchased from Pasteur Institute of Iran,Tehran,Iran.The cells were cultured in RP-MI-1640 and then supplemented with 10%(v/v) FBS and antibiotics (100 U/m L penicillin and 100?g/m L streptomycin) at 37℃in a humid environment with5%CO2.Afterward,CFS-labeled PBMCs (1×10[6]cells/well) were co-cultured with He La cells at different ratios (1∶1,1∶5,1∶10) and treated with or without various concentrations of APS by at 37℃with 5%CO2.PHA-treated PBMCs were then compared with tests (a positive control) and untreated PBMCs (negative controls).Following the co-culture for 3 d,PBMCs were separated from the supernatants,and proliferation of CFSE-labeled cells was measured via flow cytometry based on diverse fluorescence intensities of CFSE.Each extract and control were assayed in triplicate order.
Analysis of tregs induction by flow cytometry
The Treg cell population expresses CD4 as well as high levels of CD25 and low CD127.In this respect,PBMCs (1×10[6]cells/well) were co-cultured with HeLa cells using different ratios (1∶10,1∶50,1∶100)in 24-well plates and then treated with (1000?g/mL)or without APS at 37℃with 5%CO2.After 7 d,PBMCs were harvested and stained for extracellular CD4,CD25,and CD127 markers using anti-human CD4-FITC,anti-human CD25-APC,and anti-human CD127-PE monoclonal antibodies based on manufacturer's instructions.For flow cytometry analysis,the cells were then washed and re-suspended in staining buffer.The frequency of CD4+CD25+CD127-/low cells was also detected using FACS caliber (BD Bioscience).In this respect,50000 total events were collected and data analysis was fulfilled via FLOWJO software.
Analysis of MDSC induction by flow cytometry
In the present study,the MDSCs were immunophenotyped as CD33+CD11b+HLA-DR cells in PBMCs of healthy donors co-cultured with He La cells with and without being treated with APS.In brief,freshly isolated PBMCs (1×10[6]cells/well) were co-cultured with He La cells for one week at different ratios (1∶10,1∶50,1∶100) in 24-well plates with (1000?g/m L) and without APS at 37℃with 5%CO2.PBMCs,cultured in medium alone,were equally run as induction of negative control.After one week,PBMCs were collected from He La/PBMCs co-culture medium and were stained on ice for 30 min with anti-human CD33-APC,CD11b-APC-Cy7,and HLA-DR-PE monoclonal antibodies based on the manufacturer's instructions.Samples were then washed two times,and re-suspended in FACS buffer for analysis.The frequency of CD33+CD11b+HLA-DR-cells was detected using FACS caliber.
Cytokine Determination
PBMCs (5×10[5]cells/well) were co-cultured with Hela cells (1×10[5]cells/well) by using a 24-well tissue culture plate and then treated with 1000?g/m L APS or PHA (1%v/v) and also without any treatment.Moreover,PBMCs and Hela cells were cultured in a separate well and treated with 15 and 1000?g/m L APS,for PBMCs and Hela cells,respectively.As additional controls,PBMCs and Hela cells were cultured in a separate well without any treatment.The TGF-β,IL-10,IL-6,IFN-γ,and VEGF-A levels in culture supernatants were detected by ELISA after 24 h and 72 h.Diluted supernatants were then tested in a triplicate order.The absorbance was measured at 450 nm wavelength using an ELISA reader.In the test samples,cytokine concentrations were also calculated according to standard curves.
Statistical analysis
To assess differences between data groups,one-way analysis of variance (ANOVA) followed by the Mann-Whitney U test with a P<0.05 level of significance was used.All the statistical analyses were fulfilled via Graph Pad Prism software (version 8.0.2)(San Diego,CA,USA).The results are represented as mean±standard error of mean.P values less than 0.05,0.01,and 0.001 were considered statistically significant.
RESULTS
Effect of APS on PBMCs and hela cells viability
Initially,the impacts of APS on PBMCs and He La cells viability were examined.These cells were exposed to 15,30,60,120,250,500,and 1000?g/m L of APS for 24,48,72,and 96 h in normal conditions.As illustrated in Table 1,a significant increase was observed in cell viability of PBMCs following treatment with15?g/m L of APS for 96 h (P<0.05).Accordingly,PBMCs treated with APS at 15 and 60?g/m L for 24h,respectively,showed increase (Table 1) in cell viability compared with that in the control group (n=3,P<0.01 and P<0.05;respectively).According to Table 1,results demonstrated that the cell viability of He La cells had significantly decreased compared with the control group when these cells were treated with 250and 1000?g/m L of APS for 48 hrs (n=3,P<0.05and P<0.01;respectively).APS could also inhibit the growth of He La cells based on concentration and time.No toxic concentrations of APS were chosen to investigate the ability of these extracts to stimulate the proliferation of PBMCs using CFSE dye dilution assay.
Increased proliferation of PBMCs by APS in vitro
To determine the effects of APS on the proliferation of PBMCs co-cultured with He La cells,the proliferation of cultured CFSE-labeled PBMCs treated with and without concentrations of APS (15 to 1000?g/m L)was initially assessed in the absence of He La cells for4 d.As shown in Figure 1,the proliferation of PBMCs was significantly increased as the cells were treated with15?g/m L of APS for 96 h compared (Figure 1C) with untreated control (Figure 1B)(PBMCs were cultured in complete media alone)(n=4,P<0.001).
Proliferation effect of APS-treated PBMCs co-cultured with He La cells
To examine the effects of APS on the proliferation of PBMCs co-cultured with the He La cell line,the impact of He La cells on the proliferation of PBMCs was initially determined.CFSE-labeled PBMCs were col-lected after 4 d of being co-cultured with He La cells and assessed by FACS analysis.As depicted in Figure 2,the results indicated that PBMCs proliferation was significantly increased as the cells had been co-cultured with He La cells (Figure 2D) compared with naturally proliferated PBMCs (Figure 2C)(n=4,P<0.01).To examine the impact of APS on proliferated PBMCs co-cultured with He La cells,the proliferation of APS-treated PBMCs after being co-cultured with He La cells was assessed via flow cytometry.As presented in Figure 2,APS could significantly enhance the proliferation of PBMCs co-cultured with He La cells and treated with 1000?g/m L of APS (Figure 2E) for4 d compared to that in the control group (n=4,P<0.05).
Table 1 MTT assay
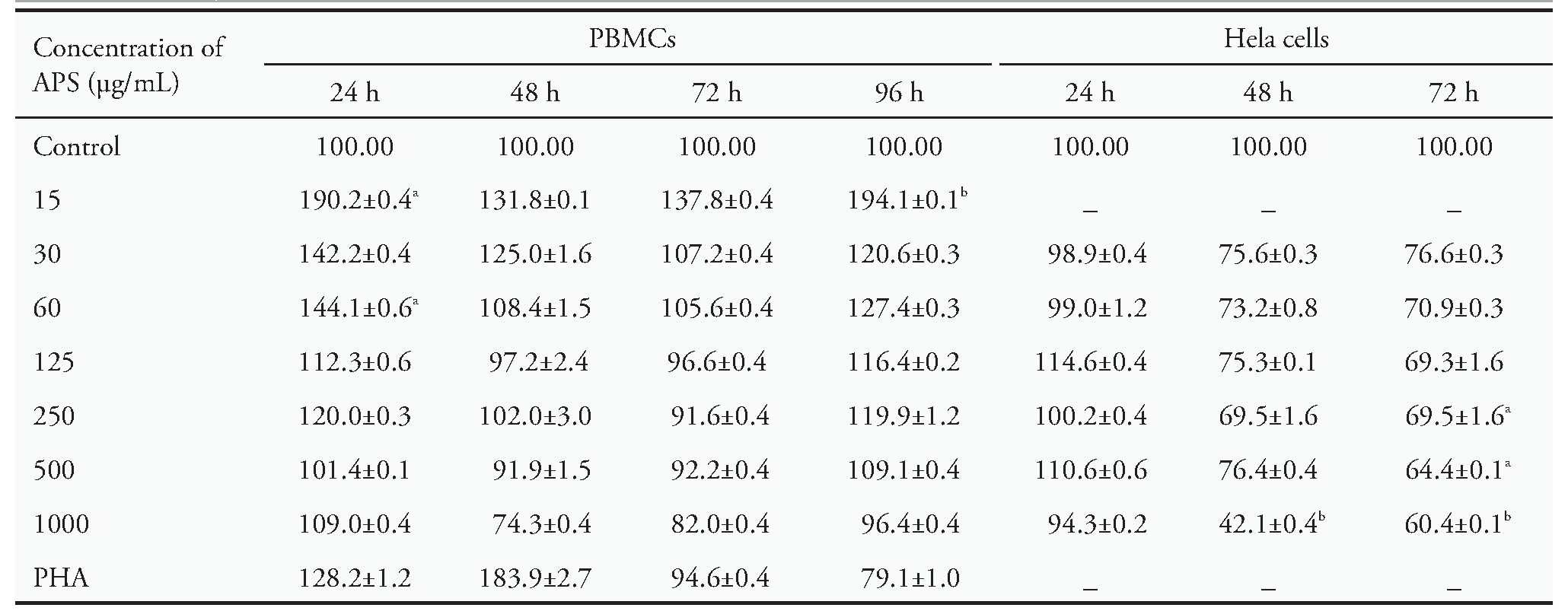
Notes:the effects of different concentrations of APS on the viability of PBMCs and He La cells.Results indicated that APS could increase the viability of PBMCs at concentrations of 15 and 60?g/m L.APS at 500?g/m L also showed minor toxicity on He La cells and reduced cell viability by around 50%at 1000?g/m L.MTT:3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide;APS:Astragalus poly-saccharides;PBMCs:peripheral blood mononuclear cells.Data are represented as mean±standard error of mean.aP<0.05,bP<0.01 vs control group.
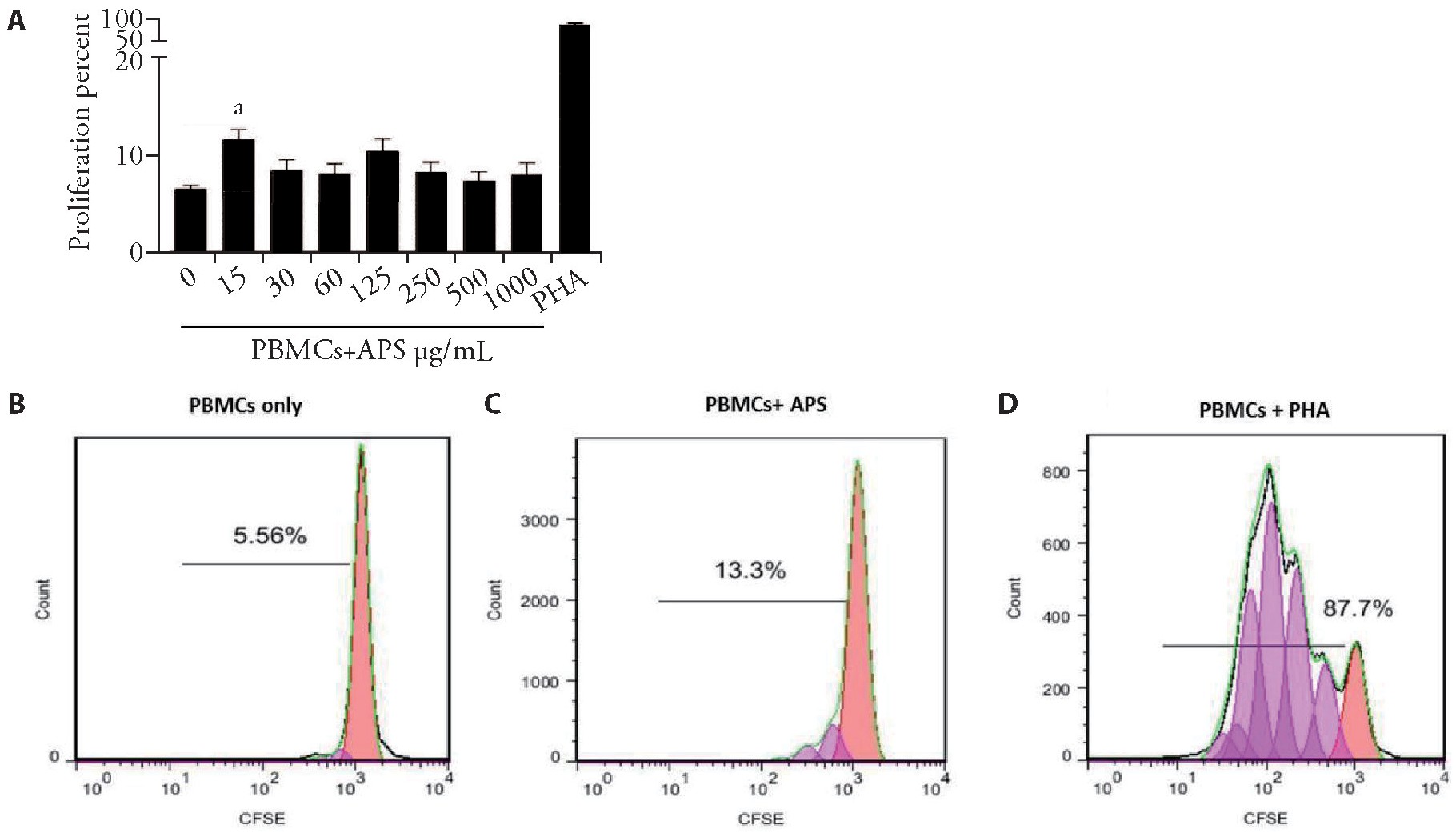
Figure 1 Proliferation of CFSE-labeled PBMCs cultured with and without APS
A:statistical analysis demonstrated significant proliferation of PBMCs in treatments with 15μg/mL of APS (n=4,P<0.001);B-D:representative histograms of CFSE dilution showed the percentage of proliferated PBMCs (C,D) and undivided control cells (B).APS:Astragalus polysaccharides;PBMCs:peripheral blood mononuclear cells;CFSE:carboxyfluorescein succinimidyl ester.Data are represented as mean±standard error of mean.aP<0.001 vs control group.
Effect of APS on induction of Treg cells
To assess whether co-cultured PBMCs and Hela cells with APS could affect the induction of Treg cells,inducing capability of PBMCs treated with 15?g/m L of APS and cultured in the absence of He La cells for7 d was determined.Following 7 d of culturing,induction of CD4+CD25+CD127-/low Treg cells was analyzed using flow cytometry.The CD4+CD25+CD127-/low Treg cell frequency in the freshly isolated PBMCs of healthy donors was also examined(Day 0) by flow cytometry.The data shown in Figure 3 indicated a significant fall in the induction of Treg cells as PBMCs treated with 15?g/m L of APS(Figure 3F,3G) compared with the control group(Figure 3E)(n=3,P<0.05).
Modulatory effect of APS on induction of Treg cells co-cultured with He La cells
A question was addressed whether co-cultured of PBMCs and Hela cells would result in the induction of Tregs or not.So,PBMCs were initially co-cultured with Hela cells in a contact-dependent manner in a ratio of 1∶50 (HeLa∶PBMCs) to assess the effect of Hela cells on the induction of Treg cells.Following 7 d of co-culture,induction of Treg cells was analyzed via flow cytometry,as illustrated in Figure 4.Co-culture of PBMCs with HeLa cells indicated a significant increase in frequency of Treg cells (Figure 4A,4D) compared with naturally occurring ones (Figure 4A,4C)(n=3,P<0.05).Then,Hela cells were co-cultured with PBMCs and treated with 1000?g/mL of APS.Following 7 d of co-culture,induction of Treg cells was analyzed by using flow cytometry.It was observed that the expansion of CD4+CD25+CD127-/low Treg cells was not significantly different in APS-treated PBMCs co-cultured with Hela cells(Figure 4E) in comparison with untreated control (Figure 4D)(n=3,P>0.05).
Effect of APS on MDSC induction
To find whether APS was involved in the induction capability of MDSCs,PBMCs were cultured alone or co-cultured with He La cells,with or without APS for7 d.Following 7 d of culture,induction of CD33+CD11b+HLA-DR-cells was analyzed via flow cytometry.The CD33+CD11b+HLA-DR-cells frequency in the population of PBMCs in healthy donors was also examined (Day 0)(Figure 5B) through flow cytometry.
Figure 2 Effect of APS on the proliferation of PBMCs co-cultured with He La cells
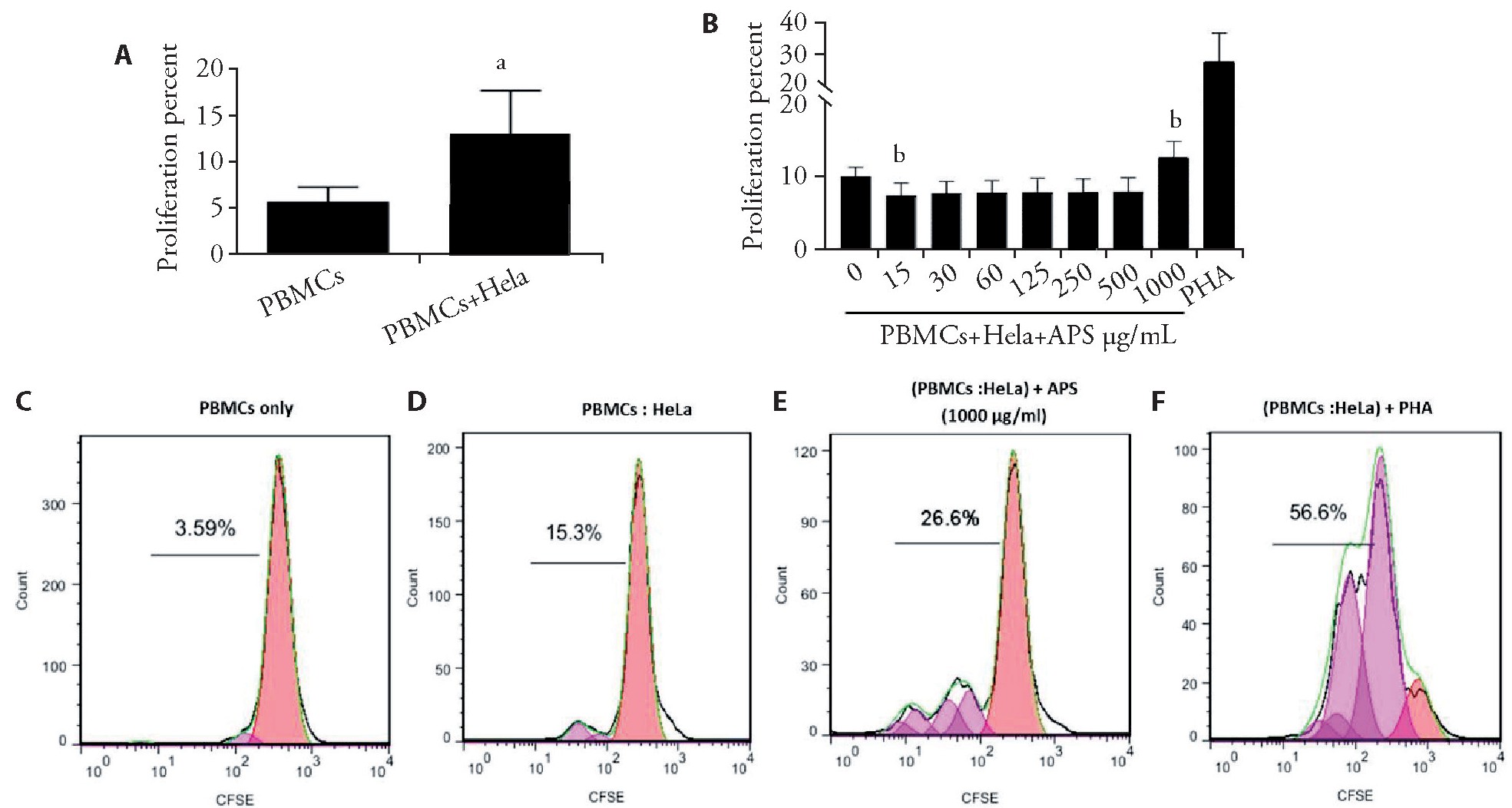
A:statistical analysis of the proliferated cells demonstrated significant proliferation of PBMCs co-cultured with HeLa cells (n=4,P<0.01);B:the proliferation of CFSE-labeled PBMCs was analyzed by using flow cytometry after being co-cultured with HeLa cells and treated with APS (n=4,P<0.05);C-F:representative histograms of CFSE dilution demonstrating the percentage of proliferated PBMCs;E:the most significant enhancing effect of APS was detected after being treated with 1000μg/mL of APS for 4 d.APS:Astragalus polysaccharides;PBMCs:peripheral blood mononuclear cells;CFSE:carboxyfluorescein succinimidyl ester.Data are represented as mean±standard error of mean.aP<0.01,bP<0.05 vs control group.
Figure 3 APS decreased the induction of Tregs in vitro
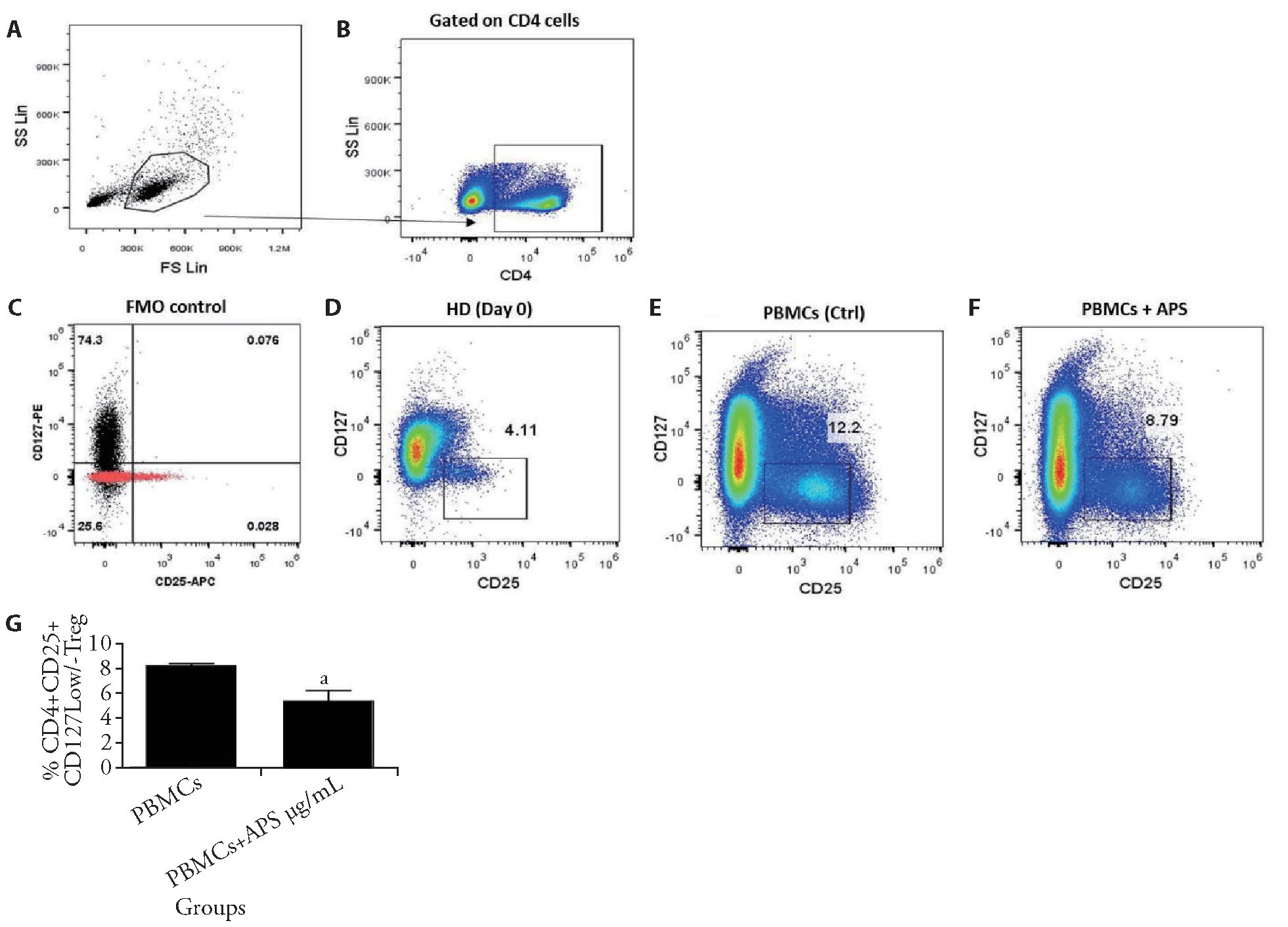
A-C:gating strategy to assess the Tregs population through flow cytometry;F:expansion of CD4+CD25+CD127-/low Tregs from PBMCs of healthy donors as treated with APS;D-F:results demonstrated the frequency of Tregs after treating with 15μg/mL of APS for 7 d compared to the control group and day 0;G:expansion of CD4+CD25+CD127-/low Tregs was assessed after PBMCs were cultured in CM alone and treated with 15μg/mL of APS.APS:Astragalus polysaccharides;PBMCs:peripheral blood mononuclear cells.Data are represented as mean±standard error of mean.aP<0.05 vs control group.
Figure 4 Proportions of CD4+,CD25+,CD127-/low Tregs from APS-treated PBMCs co-cultured with He La cells
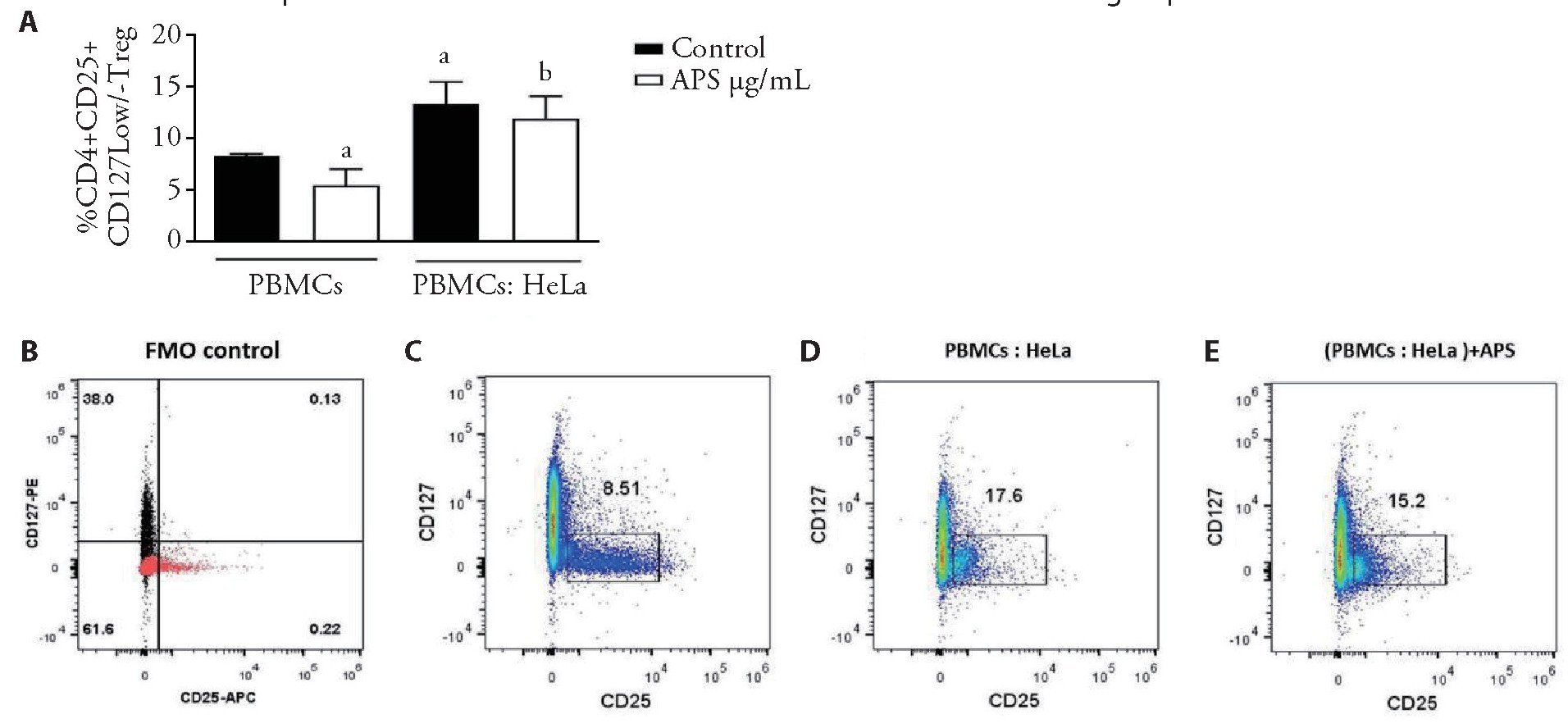
A:the statistical graph illustrated the proportion of CD4+CD25+CD127-/low Tregs induced from PBMCs co-cultured with HeLa cells and treated with APS;C-E:plots of representative flow cytometry showed the production of CD4+CD25+CD127-/low Tregs from PBMCs alone,co-cultured with He La cells and without any treatment and treated with APS.APS:Astragalus polysaccharides;PBMCs:peripheral blood mononuclear cells,b:non-sinificant.Data are represented as mean±standard error of mean.aP<0.05 vs control group.
As shown in Figure 5,no significant decrease was observed when PBMCs was cultured alone and treated with 15?g/m L of APS(Figure 5E,5C) in comparison with untreated control(Figure 5C)(n=3,P>0.05).Then,Hela cells were initially co-cultured with PBMCs in a contact-dependent manner to evaluate the impact of Hela cells on induction of MDSCs from the population of PBMCs.Accordingly,a significant rising trend was observed in the MDSCs frequency (Figure5E,5C) compared with that in the control group (Figure 5C).Therefore,the co-culture of PBMCs and He La cells revealed a significant increase in induction of MDSCs compared with naturally occurring MDSCs(n=3,P<0.05).Moreover,no significant decrease in induction of MDSCs was reported when PBMCs and He La cells co-cultured and being treated with 1000?g/m L of APS (Figure 5E,5D),compared to the control group (Figure 5D)(n=3,P>0.05).
Figure 5 CD33+CD11b+HLA-DR-cells proportions from PBMCs treated with APS and co-cultured with He La cells
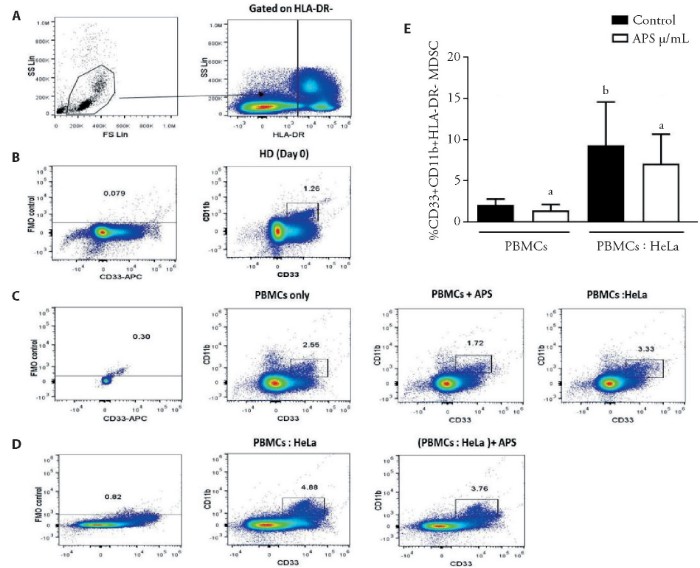
A:gating strategy to assess the population of MDSCs via flow cytometry;C,D:plots of representative density from the expansion of CD33+,CD11b+,HLA-DR-cells from PBMCs cultured alone,and treated with APS,co-cultured with HeLa cells,and treated with APS;E:Analysis of the MDSCs percentage among the PBMCs cultured alone and treated with APS as well as after co-culturing with HeLa cells.PBMCs:peripheral blood mononuclear cells;APS:Astragalus polysaccharides;MDSCs:myeloid-derived suppressor cells,a:non-significant.Data are represented as mean±standard error of mean.bP<0.05 vs control group.
Impact of APS on cytokine production
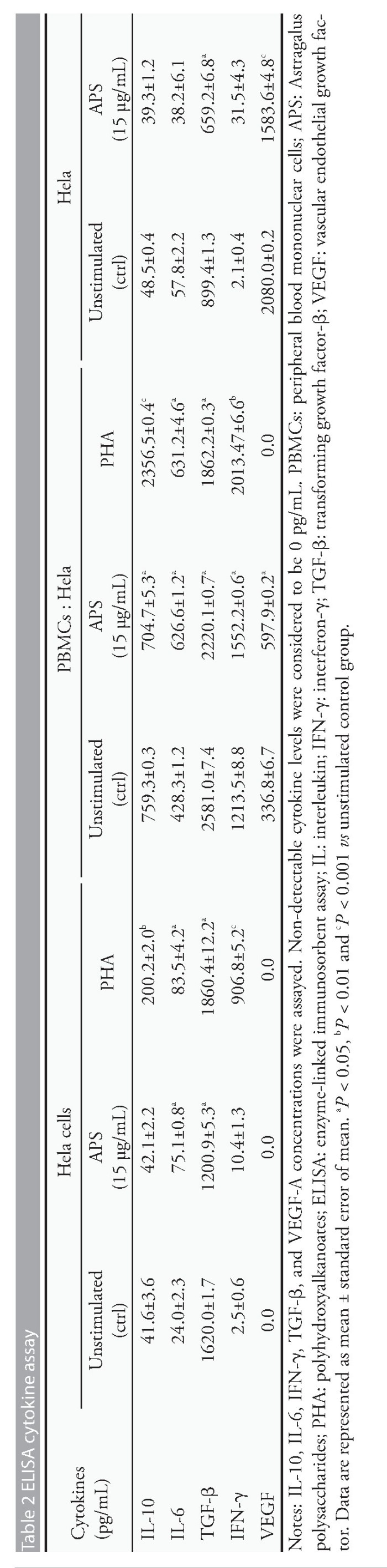
Since APS showed proliferation effect on PBMCs and suppressing effects on induction of Treg cells in vitro,the changes of cytokine production by PBMCS/He La cells that were cultured alone or co-cultured together,were investigated with or without APS.The mean level of IL-6 was significantly higher in the APS-treated PBMCs monoculture group (15?g/m L) in comparison with the untreated group (P<0.05).Besides,the concentrations of IL-6,IFN-γ,and VEGF-A were significantly higher in APS-treated PBMCs (1000?g/m L) co-cultured with He La cells in comparison with the untreated control group (P<0.05).In contrast,the concentration of IL-10 and IFN-γdid not vary between the APS-treated PBMCs monoculture group and the untreated control one (P>0.05).In addition,the production of IL-10 and TGF-βwas significantly lower in APS-treated PBMCs co-culture with He La cells in comparison with the untreated group (P<0.05).After 72 hrs exposure to APS,He La cells had significantly released less TGF-βand VEGF-A levels compared with untreated He La cells (P<0.05 and P<0.001,respectively;Table 2).The results of this study clearly showed that APS might have immunomodulatory activity by modifying the secretion of cytokines in TME (Table 2).
DISCUSSION
Traditional Chinese Medicine (TCM) has been the most useful therapeutic method in china for thousands of years.Polysaccharides as one of the active and impor-
tant components of TCM with various biological activities are becoming a common remedy for cancer patients since they can enhance immune system potentials against tumor cells.[13,14]Among the different origins of APS,the active component obtained from Chinese medicinal herb (i.e.astragalus membranaceus) has exhibited various biological activities,like immunomodulatory effects.[15,16,17]At present,numerous studies have indicated that APS can increase therapeutic effects and reduce adverse ones,and consequently contribute to improved immune responses in vivo.[6,18]However,little has been reported regarding APS potential in boosting immune system ability against tumors.The results of this study indicated that APS could modulate immune responses in vitro.
The proliferation of lymphocytes is the most direct indicator of signaling during cellular immune responses.[19]Previous studies have demonstrated that APS can have immunomodulatory effects,including enhanced proliferation activities of lymphocytes and augmented TLR4-mediated My D88-dependent signaling pathway activation.[6,20,21,22,23,24]Furthermore,numerous studies have shown that TLRs can regulate the proliferation of cells to multiply beneficial immune responses against tumors.[15,25,26]Moreover,IL-6 is considered as a cytokine with several immunomodulatory functions that can stimulate the proliferation of T cells,B cells,and stem cells,consequently promoting immunoglobulin production by B cells.[16,27]In this respect,Tian et al[28]reported that APS had an anti-tumor effect combined with Adriamycin in vivo due to its capacity to enhance IL-6 and tumor necrosis factor (TNF)-αexpression in H22 tumor-bearing mice.In other investigations,IFN-γwas reported to have a significant anti-tumor effect via modulating tumor cells,immune cells,and/or non-immune stromal ones in TME.[29]Another report suggested that APS could up-regulate T-bet m RNA expression and concentrations of IFN-γin individuals with CC as well as similarly in lung cancer mice models.[16,30]Another report also implied an explicit role of IFN-γon non-stimulated CD4+T cells that could boost adaptive immunity through augmenting survival in the course of initiation of immune responses.[31]These experimental results implicated that APS could boost the proliferation of PBMCs,and secretion of IL-6 and IFN-γ,which was in line with these experimental results in a co-culture model.Moreover,the results of our study suggested that the impact of APS on proliferated PBMCs might be related to its ability to enhance IL-6 expression.Nonetheless,the precise molecular mechanism and role of innate immunity receptors require much more explanation.
It should be noted that Treg cells can also suppress anti-tumor immune responses and be contributing to the development of immunosuppressive TME in cancer types.[32]A previous report revealed that malignant plasma cells might lead to a significant induction of CD4+CD25+Fox P3+Treg cells based on a contact-dependent manner[33]which was consistent with the experimental results in our study.
Other studies had also suggested the inhibitory role of APS on the frequency of Tregs and expression of Forkhead box P3 both in vivo and in vitro.[34,35,36]Furthermore,astragalosideⅣ(AS-Ⅳ) could inhibit tumor progression in a murine lung tumor model via down-regulating the percentage of Treg cells in vitro and in vivo.[37]The results of the present study were in agreement with this report.TGF-β1 and IL-10,as inhibitory cytokines,are also involved in the suppressive functions of Treg cells and may inhibit effector T cell proliferation.[38]APS has been able to modify responses through immunomodulatory activities to repress the production of IL-10 from cultured macrophages in a dose-dependent manner in vitro.[39]Likewise,it has been reported that TGF-βexpression and Treg cell frequency were down-regulated following administration of APS in vivo.[35]In the present study,the production of TGF-βand IL-10 from PBMCs co-cultured with He La cells was decreased and the induction of Treg cells was inhibited through APS in monoculture of PBMCs.But,induction of Treg cells had no significant change when PBMCs and He La cells were co-cultured and treated with APS.It is assumed that the immunomodulatory effects of APS in various cancer types might be different.However,there were no relevant reports in this case.Therefore,it is suggested to examine the complete molecular mechanism of this phenomenon as well as the impact of APS on the Treg cell function in future studies.
Here,we also explored the capacity of APS on MDSC induction as well as the relationship between APS-treated cells and VEGF production.It should be noted that MDSCs are included in the immune escape of cancer cells,hence they can promote an uncontrolled growth of tumors.[40]Recently,Guo et al[41]reported that radix astragali had an inhibitory function in MDSCs proliferation and could also prevent MDSCs induced by TGF-βexpression and CD8+T cell apoptosis.It is now apparent that angiogenesis is one of the key factors in tumor development.VEGF also augments the generation of new capillaries that provide oxygen and nutrients to tumors.[42]A previous study indicated that the protein level of VEGF was decreased in an APS-treated group in hepatoma Hep G2-bearing mice.[43]In addition,AS-IV could reduce VEGF expression in an orthotropic nude-mouse model of human hepatocellular carcinoma.[44]It has been confirmed that VEGF plays a leading role in MDSC expansion and function.[45]Our data indicated that APS could decrease VEGF-A production by He La cells in vitro,but not in the co-culturing assay.Thus,it seems that APS can directly improve anti-tumor responses via overcoming inhibitory effects of MDSCs as well as regulating the secretion of immunomodulatory factors in vitro as well as down modulating the VEGF production.Meanwhile,the underlying biological mechanisms employed by APS to inhibit VEGF production have been underestimated.Nevertheless,results of MDSC induction and VEGF production indicated no significant changes when PBMCs and He La cells were co-cultured and treated with APS.Therefore,APS is highly likely to suppress inhibitory functions of MDSC instead of inhibiting MDSC expansion.However,no relevant reports have been divulged and further studies are required to account for this issue.
In conclusion,APS showed significant immunomodulatory activities,including suppression of Treg cell induction,promotion of TGF-βIL-10 production by PBMCs,and increased production of inflammatory cytokines IL-6 and IFN-γin vitro.These observations suggest that APS confers a significant impact on stimulating an immune-mediated response against tumor cells.As a consequence,APS might enhance anti-tumor effects by regulating immune responses as a scientific foundation for the development of clinical research protocols to test these data in vivo and provide an insight into anti-cancer therapies.
参考文献
[1] Ferlay J,Soerjomataram 1,Dikshit R,et al.Cancer incidence and morality worldwide:sources methods and major patterns in GLOBOCAN 2012.Int J Cancer 2015;136(5);:359-38
[2] Arbyn M.Attila A,Jordan J,et al.European guidelines for quality assurance in cervical cancer screening-summary document. Ann Oncol 2010;21(3):448-458.
[3] Dhama K,Saminathan M,Jacob SS,et al.Effect of immunomodulation and immunomodulatory agents on health with some bioactive principles,modes of action and potent biomedical applications Int J Pharmacol 2015;11(4):253-290.
[4] Vinay DS,Ryan EP,Pawelec G,et al,editors. Immune evasion in cancer.mechanistic basis and therapeutic strategies. Semin Cancer Biol 2015: Elsevier.
[5] Fleming V,Hu X,Weber R,et al. Targeting myeloid-derived suppressor cells to bypass tumor-induced immunosuppression. Front Immunol 2018;9:398.
[6] Zhou L,Liu Z,Wang Z.et al.Astragalus polysaccharides exerts imunomodulatory efects via TLR4-mediated My D88-dependent signaling pathway in vitro and in vivo.Sci Rep2017;7:44822.
[7] Shahrajabian MH,Sun W,Cheng Q Astragalus, an ancient medicinal root in Traditional Chinese Medicine,a gift from Silk Road.Int J Agric Biol Sci 2019;3:27-38.
[8] Liu P,Zhao H,Luo Y.Anti-aging implications of Astragalus Membranaceus (Huangqi):a well-known chinese tonic. Aging Dis 2017;8(6):868.
[9] Cho WC,Leung KN.In vitro and in vivo anti-tumor efects of Astragalus membranaceus .Oncol Lett 2007;252(1):43-54.
[10] He X,Niu X,Li J,Xu S,Lu A.Immunomodulatory activities of five clinically used Chinese herbal polysaccharides. JExp Integr Med 2012;2(1):15-27.
[1] Cho WC,Leung KN.In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J Ethnopharmacol 2007;113(1):132-141.
[12] Jin M,Zhao K,Huang Q Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus.Int J Biol Macromol 2014;64:257-266.
[13] Nurmamat E Xiao H,Zhang Y,Jiao Z Effects of diferent temperatures on the chemical structure and antitumor activities of polysaccharides from cordyceps militaris .Polymers(Basel) 2018;10(4)-430.
[14] Yu J,Ji HY,Liu AJ .Alcohol-soluble polysaccharide from Astragalus membranaceus:preparation,.characteristics and antitumor activity.Int J Biol Macromol 2018;118:2057-2064.
[15] Reynolds JM,.Martinez GJ,Chung Y,Dong C. Tl-lile receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA 2012;109(32): 13064-13069.
[16] Zhou X,Liu Z,Long T,Zhou L,Bao Y.Immunomodulatory effects of herbal formula of astragalus polysaccharide(APS) and polysaccharopeptide (PSP) in mice with lung cancer.Int J Biol Macromol 2018;106:596-601.
[17] Wang J,Jia J,Song L,et al. Extraction,structure and pharmacological activities of Astragalus Polysaccharides Appl Sci 2019;9(1):122.
[18] Zhao B,Liu H,Zhang H.,et alAstragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting theexpression of transforming growth factorBand the frequency of regulatory T cells. FEMS Immunol Med Microbiol 2011;63(2):228-235.
[19] Michaud DS,Houseman EA,Marsit CJ,Nelson HH,Wiencke JK,Kelsey KT.Understanding the role of the immune system in the development of cancer.new opportunities for population-based research. Cancer Epidemiol Biomarkers Prev 2015;24(12):1811-1819.
[20] Shao BM,Xu W,Dai H,Tu P,Li Z,Gao XM.A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus,a Chinese medicinal herb. BiochemBiophys Res Commun 2004;320(4)110-1111.
[21] Sun s,Zheng K,Zhao H,et al.Regulatory effect of astragalus polysaccharides on intestinal intraepithelialyδT cells of tumor bearing mice .Molecules 2014; 19(9): 15224-15236.
[22] Zhong R,Yu M,Liu H,Sun H,Cao Y,Zhou D.Effects of dietary Astragalus polysaccharide and Astragalus membranaceus root supplementation on growth performance,rumen fermentation,immune responses,and antioxidant status of lambs .Anim Feed Sci Technol 2012;174(1-2):60-67.
[23] Zhu ZY,Zhang JY,Liu F,Chen L,Chen LJ,Tang Y.Characteriz ation and lymphocyte proliferation activity of an oligosaccharide degraded from Astragalus polysaccharide.Med Chem Comm 2017;8(7):1521-1530.
[24] Guo L,Wang D,Hu Y,et al.Adjuvanticity of compound polysaccharides on chickens against Newcastle disease and avian influenza vaccine Int J Biol Macromol 2012;50(3):512-517.
[25] Li X,Jiang S,Tapping RIOll-like receptor signaling in cell proliferation and survival. Cytokine 2010;49(1):1-9.
[26] Schweighoffer E,Nys J,Vanes L Smithers N.Tybulewicz VL.TLR4 signals in B lymphocytes are transduced via the B cell antigen receptor and SYK.J Exp Med 2017;214(5):1269-1280.
[27] Akira S, Hirano T,Taga T,Kishimoto T. Biology of multifunctional cytokines:IL-6 and related molecules (IL 1and TNF).FASEB J 1990;4(11)-.2860-2867.
[28] Tian QE,Li HD,Yan M,Cai HL,Tan QY,Zhang WY.Astragalus polysaccharides can regulate cytokine and P-glycoprotein expression in H22 tumor-bearing mice.World JGastroenterol 2012;18(47):7079-7086.
[29] Mojic M, Takeda K,Hayakawa Y.The dark side of IFN-gamma:its role in promoting cancer immunoevasion.Int J Mol Sci 2017;19(1):89.
[30] Hu YJ,Li L,Gong SX.Regulatory effect of astragalus injection on Th1/Th2 cell function in patients with cervical cancer. Zhong Guo Zhong Xi Yi Jie He Za Zhi 2010;30(11):1157-1159
[31] Reed JM,Branigan PJ,Bamezai A. Interferon gamma enhances clonal expansion and survival of CD4+T cells J Interferon Cytokine Res 2008;28(10):611-622.
[32] Chaudhary B, Elkord E Regulatory T cells in the tumor microenvironment and cancer progression:role and therapeutic targeting. Vaccines (Basel) 2016;4(3):28.
[33] Feyler S,Scott GB,Parrish C,et al.Tumour cell generation of inducible regulatory T-cells in multiple myeloma is contact-dependent and antigen-presenting ell-independent.PL0 s One 2012;7(5):e35981.
[34] Hou YC,Wu JM,Wang MY,et al.Modulatory effects of Astragalus polysaccharides on T-cell polarization in mice with polymicrobial sepsis. Mediators Inflamm 2015;2015:82631
[35] Du X,Chen X,Zhao B,et al.Astragalus polysaccharides enhance the humoral and cellular immune responses of hepatitis B surface antigen vaccination through inhibiting the expression of transforming growth factorβand the frequency of regulatory T cells FEMS Immunol Med Microbiol 2011;63(2):228-235.
[36] Li Q,Bao JM,Li XL ,Zhang T,Shen XH.Inhibiting effect of Astragalus polysaccharides on the functions of CD4+CD25 high Treg cells in the tumor microenvironment of human hepatocellular carcinoma Chin Med J(Engl) 2012; 125(5):786-793.
[37] Qi Y,Gao F,Hou L,Wan C .Anti-Inflammatory and Immunostimulatory Activities of Astragalosides Am J Chin Med 2017;45(6)-1157-1167.
[38] Schmitt EG,Williams CB Generation and function of induced regulatory T cells .Front Immunol 2013;4:152.
[39] Clement-Kruzel S, Hwang S-A,Kruzel MC,Dasgupta A,Actor JK Immune modulation of macrophage pro-inflammatory response by goldenseal and Astragalus extracts ,JMed Food 2008;11(3):493-498.
[40] Salminen A, Kaarniranta K, Kauppinen A. Phytochemicals inhibit the immunosuppressive functions of myeloid-derived suppressor cells (MDSC):impact on cancer and age-related chronic inflammatory disorders. Int Immunopharmacol 2018;61:231-240.
[41] Guo Q,Li J,Lin H.Effect and molecular mechanisms of Traditional Chinese Medicine on regulating tumor immunosuppressive microenvironment Biomed Res Int 2015;2015.261620.
[42] Duffy AM, Bouchier-Hayes DJ,Harmey JH.Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells :autocrine signalling by VEGF. Madame Curie Bioscience Database 2004:133- 144.
[43] Su J,Jiang L,Wu J,Liu Z,Wu Y. Immunologic effect of polysaccharides extracted from sipunculus nudus (SNP)onhepatoma Hep G2-bearingmice .bio Rxiv2017:175190.
[44] Zhang S,Tang D,Zang W,et al. Synergistic inhibitory effect of Traditional Chinese Medicine astragalosideIVand curcumin on tumor growth and angiogenesis in an orthotopic nude-mouse model of human hepatocellular carcinoma Anticancer Res 2017;37(2):465-473.
[45] Umansky VBlattner C,Gebhardt C.Utikal J.The role of myeloid-derived suppressor cells (MDSC) in cancer progression.Vaccines (Basel) 2016;4(4):36.