摘 要: 神经嵴 (neural crest, NC) 是一种具有高度迁移能力的多功能细胞群, 它形成于胚胎发育过程中神经上皮和上皮细胞前体之间的交界处。神经嵴细胞在经历了横贯整个胚胎的迁移之后, 会固定下来并分化发育成多种组织和器官。神经嵴细胞在迁移过程中表现出趋化性 (chemotaxis) 和趋电性 (electrotaxis) 。神经嵴细胞能够沿着胞外可溶性因子浓度梯度产生定向迁移, 这些趋化性因子包括SDF-1、VEGE、FGF、PDGF等;神经嵴细胞也能在生理电场 (endogenous electric fields) 或适当外源电场 (exogenous electric fields) 中沿电场方向, 向正极或负极迁移。一些重要的与趋电性相关的分子已经被发现, 如EGFR、Rac1、V-ATPase H+pump、PI3 kinase/Pten。本综述详细介绍了神经嵴细胞趋化性和趋电性迁移中的可能机理和实验证据, 为后续研究提供参考。
关键词: 神经嵴; 细胞迁移; 趋化性; 趋电性;
Abstract: Neural crest is a kind of multipotent cell population with high migration ability. It is formed at the interface between the neuroepithelium and the prospective epidermis during embryonic development. After the migration across the entire embryo, the neural crest cells were fixed and differentiated into a variety of tissues and organs. And the neural crest cells showed chemotaxis and electrotaxis during the migration. Neural crest cells could make directional migration along the extracellular concentration gradients of soluble factors. These chemotaxis factors included SDF-1, VEGE, FGF, and PDGF. Neural crest cells could directionally migrate toward either anode or cathode in endogenous electric fields or exogenous electric fields. And several critical molecules related to electrotaxis havex been discovered, including EGFR, Rac1, V-ATPase H+pump, and PI3 kinase/Pten.This review introduced the possible mechanisms and experimental evidence for chemotaxis and eletrotaxis during neural crest cell migration in detail, which might provide references for the follow-up study.
Keyword: Neural crest; Cell migration; Chemotaxis; Electrotaxis;
神经嵴 (neural crest, NC) 是一个仅在脊椎动物胚胎中短暂出现的细胞群体。它最初是由外胚层神经板和上皮细胞在神经板边界相互作用时诱发形成的。神经褶的融合诱导形成一个闭合的神经管并且两侧富含神经嵴细胞 (Theveneau and Mayor, 2012;Duband et al., 2015) , 同时, 神经板和神经上皮也可以参与神经嵴的形成。诱导结束后, 神经嵴细胞经历了从上皮细胞到间充质细胞的转换 (epithelial-to-mesenchymal transition, EMT) , 产生一些新特性:细胞获得自主运动性, 细胞极性消失并获得一个钙粘素的表达开关 (Nieto, 2013) 。通常这些变化伴随细胞骨架的变化, 这就意味着神经嵴细胞脱离了位于神经管背部的神经上皮, 从而使得细胞本身具有很高的机动性 (Theveneau and Mayor, 2012) 。神经嵴细胞能分化为多种类型的细胞进而形成多种组织器官, 可以认为其是一大类多功能干细胞的集合 (Dupin et al., 2006) 。神经嵴细胞从神经管、中胚层两侧以及外胚层边缘处接受迁移诱导信号 (Rogers et al., 2012) 。这些细胞的命运由旁分泌信号决定, 一般常与它们的起源、位置以及迁移时间有关 (Shellard and Mayor, 2016) 。
1、 神经嵴细胞在胚胎中的迁移方式
细胞迁移是许多发育过程以及创伤修复的基本途径, 包括胚胎的形态发生、伤口愈合和免疫应答 (Friedl and Gilmour, 2009) 。这些过程总是涉及到细胞对一些能指导细胞迁移的特殊信号分子的响应 (Ricoult et al., 2015) 。同时, 细胞也能对一些机械刺激、电流刺激、胞外基质成分等产生响应 (Roca-Cusachs et al., 2013;Charras and Sahai, 2014;Das et al., 2015;Riahi et al., 2015) 。
在经历了从上皮细胞到间充质细胞的转换 (EMT) 后, 神经嵴细胞变成一种具有高机动性的细胞群, 其迁移和侵袭能力, 类似于恶性肿瘤 (Theveneau and Mayor, 2011) 。它们最开始呈波状移动, 离开神经管向胚胎前侧下方迁移, 但很快分裂成许多沿不同方向稳定前行的细胞流 (Kuo and Erickson, 2014) 。
神经嵴细胞迁移在许多脊椎动物中被研究过, 包括鸡、小鼠、非洲爪蟾、斑马鱼等模式生物。在鸡和小鼠的胚胎中, 早期躯干神经嵴细胞通过前端生骨节向腹外侧迁移, 生成黑色素细胞的躯干神经嵴随后在体节结构的影响下, 沿着在生皮肌节和背外胚层之间的背外侧路径迁移 (Loring and Erickson, 1987) 。而在斑马鱼和非洲爪蟾胚胎中, 生黑色素细胞则沿着腹正中和背外侧两条路径同时迁移 (Kelsh et al., 2009) 。近些年在一些非典型的模式生物中也有研究, 例如七鳃鳗、盲鳗和龟 (Gilbert et al., 2007;Ota et al., 2007, Nikitina et al., 2008;Cebra-Thomas et al., 2013;Barriga et al., 2015) 。
颅神经嵴细胞分成三条细胞流涌入分段鳃弓 (branchial arches, Bas) 这个过程在很大程度上受Eph/ephrin信号和3型信号素 (class 3 semaphorins) 影响。在鸡胚胎中, 主要是Eph/ephrin信号起到作用, 其阻止神经嵴细胞入侵没有神经嵴细胞的组织和后半部的体节, 从而将其限制在有喙的前半部体节中 (Gammill and Roffers-Agarwal, 2010;Kuo and Erickson, 2014) 。3型信号素也可以通过与神经嵴细胞表达的丝状蛋白-神经纤维网蛋白复合体作用, 将神经嵴细胞分隔于头部、躯干和尾椎阻止入侵 (Toyofuku et al., 2008) 。神经嵴细胞来源不同会导致表达的Eph/ephrin受体和配体蛋白不同, 所以来源不同细胞流的神经嵴细胞不能混合 (Kuo and Erickson, 2014) 。
不同物种的神经嵴细胞表现出不同的迁移行为, 其中有一些神经嵴细胞显示出较为独立的迁移行为 (Kulesa et al., 2004) , 但是大多数的神经嵴细胞都会成链或者成片地迁移 (Thiery et al., 2009;Kulesa et al., 2010) 。这种细胞与细胞之间产生相互作用, 形成一簇或一片细胞, 并且具有定向性的协同迁移方式被称为细胞的集体迁移。一些证据表明 (Kulesa and Fraser, 2000;Teddy and Kulesa, 2004) , 细胞在集体迁移过程中的总体定向性要高于单个细胞的定向性, 说明细胞间的相互作用可能促进细胞群体的迁移定向性。在经过从上皮细胞到间充质细胞的转换之后, 神经嵴细胞之间失去了紧密连接, 细胞与细胞之间的相互作用变得微弱, 细胞之间多依靠N-钙黏素连接在一起 (Kulesa and Mc Lennan, 2015) 。钙黏素的作用强度受溶血磷脂酸受体2 (LPAR2) 的限制, 使得神经嵴细胞组织具有较强的可塑性, 能在不消除细胞相互作用的情况下成片地进行迁移 (Kuriyama et al., 2014) 。
在早期胚胎发育过程中, 神经嵴细胞必须进入并充满一片或几片特殊的目标区域 (图1A) , 因此, 能够定向迁移对于神经嵴细胞来说至关重要。定向迁移的细胞首先需要产生极化, 使细胞前方肌动蛋白聚合, 后方肌动蛋白收缩 (Krause and Gautreau, 2014) 。而神经嵴细胞之间所产生的细胞接触抑制 (contact inhibition of locomotion, CIL) 就能使它们在接触时产生极化作用 (Roycroft and Mayor, 2016) 。在成片细胞的边缘, Rac信号被激活使细胞产生伪足, 而在细胞接触位点, 神经嵴细胞通过表达蛋白聚糖4 (syndecan-4) 和N-钙粘素信号来抑制Rac信号 (Matthews et al., 2008;Carmona-Fontaine et al., 2014) 并激活Rho A信号来减少板状伪足的产生 (图1B) (Shellard and Mayor, 2016) 。
2、 神经嵴细胞的趋化性
一个世纪前, Engelmann (1882) 和Pfeffer (1884) 第一次发现了或排斥 (Butler and Dodd, 2003) 或吸引 (Roussos et al., 2011) 细胞的因子。细胞对这些可溶性胞外因子浓度梯度的响应被称作趋化性-这是一种能很好地指导细胞定向迁移的机制 (Roussos et al., 2011;Roca-Cusachs et al., 2013) , 这其中影响细胞迁移的因子称为趋化因子 (chemoattractant) 。趋化因子通常由目标组织转录、合成和分泌, 指导特定细胞迁移至特定位置。而这些响应的细胞需要在合适的时候表达出这些趋化因子的受体。在缺失趋化因子或受体时, 细胞将被指导迁移至错误的区域或出现无定向的迁移。在体外, 局部的趋化因子能引起细胞的趋化性。而在体内, 异常的趋化因子会改变细胞的正常路径。趋化性需要细胞向高浓度的可溶性因子迁移, 因此趋化因子的浓度梯度应该是稳定且能被检测到的, 但是现有技术暂时还未能检测到胚胎体内趋化因子的浓度梯度, 不过已有研究表明在某些情况下, 趋化因子的浓度梯度是可以由迁移中的细胞自己产生 (Cai and Montell, 2014) 。
2.1、 神经嵴细胞对特定信号的响应
目前, 已经发现很多能使神经嵴细胞产生趋化性的趋化因子, 包括SDF-1/CXCL12 (Rezzoug et al., 2011) 、FGF (Sato et al., 2011) 、VEGF (Mc Lennan et al., 2015) 、PDGF (Kawakami et al., 2011) 、SCF (Rovasio et al., 2012) 、NT-3 (Zanin et al., 2013) 、GDNF (Goto et al., 2013) 、NRG1 (Saito et al., 2012) 和TGFβ (Saika et al., 2001) (表1) 。然而, 神经嵴细胞有自我组织的性质, 并在完全发育成目标组织之前就开始迁移 (Alfandari et al., 2003) 。现有研究还不清楚是什么原因使得不同的神经嵴细胞能够在互不影响的情况下共享迁移路径, 并能在几种有限的趋化因子的指导下完成复杂的迁移。尽管有一些证据支持上述信号分子能够作为神经嵴的趋化因子, 但还没有发现沿着神经嵴细胞迁移路径上哪一种分子存在浓度梯度。所以, 胚胎中趋化因子存在的必要性有待考证。
图1 神经嵴细胞Figure 1 Neural creast migration
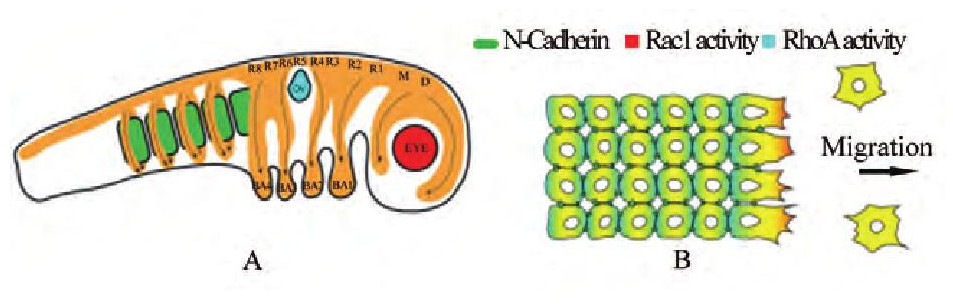
注:A:神经嵴细胞在胚胎发育过程中的迁移路径;B神经嵴细胞的极化机理 (Shellardand Mayor, 2016) ;D:间脑, M:中脑;R:菱脑原节;OV:听囊;BA:鳃弓;绿色方形:体节;橙色区域:神经嵴细胞Note:A:The pathway of neural creast migration during embryonic development;B:The mechanism of neutral crest polarity (Shellard and Mayor, 2016) ;D:Diencephalon;M:Mesenceph alon;R:Rhombomere;OV:Otic vesicle;BA:Branchial arch;Green squares:Somites;Orange area:Neural crest cells
2.1.1、 血小板衍生生长因子 (PDGF)
在不同种类的颅神经嵴非神经元衍生细胞迁移过程中多数需要血小板衍生生长因子受体α (PDGFRα) 表达 (He and Soriano, 2013) , 但在小鼠胚胎中, 心神经嵴细胞正常迁移则需要PDGFRα和PDGFRβ这两种受体的表达 (Richarte et al., 2007) 。并且也发现在小鼠胚胎的外胚层、听囊和喉内胚层中发现PDGFRα的同源配体PDGFA和PDGFC是神经嵴细胞的趋化目标 (Tallquist et al., 2000) 。斑点杂合体 (Patch heterozygotes) 就是一种删除PDGFRα编码基因导致神经嵴细胞分化成色素细胞的发育缺陷个体 (Morrison-Graham et al., 1992) 。斑点杂合体的神经嵴细胞异常发育可引起腭裂, 暗示心神经嵴细胞的缺陷和PDGERα的突变可导致发育畸形 (Kirby and Hutson, 2014) 。但是, 现阶段仍然不清楚究竟哪个配体是PDGFRβ接受信号所必须的, 以及它在体内是如何在神经嵴趋化性中起作用的。
2.1.2、 间质细胞衍生因子1 (SDF-1)
间质细胞衍生因子1 (SDF-1, 也称作CXCL12) 在胚胎发育过程中控制着许多组织的定向迁移过程, 包括斑马鱼后侧线原基细胞、原始胚芽细胞和许多神经嵴衍生细胞的迁移 (Boldajipour et al., 2008;Ding et al., 2013) 。在许多模式生物中, SDF-1是沿着神经嵴细胞表达其相应受体CXCR4的路径来表达的 (Kasemeier-Kulesa et al., 2010;Escot et al., 2013) 。虽然, 神经嵴细胞对SDF-1的趋化性活动并不总是能够被检测到, 但是还有一些实验证据能够支持细胞对SDF-1的趋化性。例如, 表达CXCR4的神经嵴细胞在体外对SDF-1有趋化性, 而SDF-1表达缺失的个体, 那些神经嵴细胞会偏离正常的路径 (Braun et al., 2002) 。转录因子HIF-1α可以通过调节CXCR4的表达来控制细胞对SDF-1的趋化性 (Barriga et al., 2013) 。
表1 神经嵴细胞趋化因子及受体Table 1 The chemotactic factors and receptors of neural crest cells

有一些实验证据表明 (Kasemeier-Kulesaetal., 2010;Saito et al., 2012) , 不同类型的神经嵴细胞对SDF-1和神经调节蛋白有不同的响应, 决定它们是迁移至交感神经中枢还是后根神经中枢。所以, Belmadani等 (2009) 推测:不同的神经嵴细胞能表达不同的受体, 使得不同的神经嵴细胞迁移至不同的区域, 并分化为不同的组织。
2.1.3、 成纤维细胞生长因子 (FGF)
FGF8在外胚层和内胚层中表达, 但它并不是神经嵴细胞表达的产物 (Abu-Issa et al., 2002;Frank et al., 2002;Walshe and Mason, 2003) 。它在小鼠胚胎中的表达依赖于Notch信号;在鸡胚胎中的表达依赖神经嵴细胞本身的存在 (High et al., 2009) 。神经嵴细胞群体迁移至目的地时所依赖的信号分子是FGF8 (Abu-Issa et al., 2002;Frank et al., 2002;High et al., 2009;Cavanaugh et al., 2015) 。然而, 有证据 (Cavanaugh et al., 2015) 表明FGF8仅对神经嵴细胞的迁移行为很重要, 而对趋化性没有影响。在小鼠和鸡胚胎中, 神经嵴细胞对FGF8的趋化性在心脏发育过程中是必不可少的 (Macatee et al., 2003;Cavanaugh et al., 2015) ;但在斑马鱼中, FGF信号对心脏的形成则是非必须的 (Cavanaugh et al., 2015) 。另一种对神经嵴细胞趋化性有影响的信号是FGF2, 它在下颌间质中受FGF8控制局部表达 (He and Soriano, 2013) 。尽管神经嵴细胞在体外对FGF2有趋化性, 但在体内还没有对FGF2的趋化性研究。
2.1.4、 血管内皮生长因子 (VEGF)
VEGF在鸟类胚胎的头部外胚层表达, R4 (Rhombomeric 4) 颅神经嵴细胞在其背外侧的迁移路径上表达VEGF的典型受体VEGFR2以及neuropilin-1 (神经毡蛋白1) (Mc Lennan and Kulesa, 2007) 。VEGF的表达随后扩展到了第二鳃弓 (BA2) 中, 而在外胚层路径中的表达减少 (Mc Lennan and Kulesa, 2010) 。在体内, R4神经嵴细胞能够被异常的VEGF信号影响, 使其偏离正常路径 (Mc Lennan and Kulesa, 2010;Mc Lennan et al., 2015) ;干扰VEGF/VEGFR2/neuropilin-1信号不会影响细胞向BA2入口的定向迁移, 但能阻止细胞接下来向BA2区域的入侵 (Mc Lennan and Kulesa, 2007;Mc Lennan and Kulesa, 2010;Mc Lennan et al., 2010) 。而在体外, 头神经嵴细胞被BA2细胞和VEGF信号吸引。
然而, 在迁移最初阶段, VEGF在外胚层中的表达是不存在浓度梯度的 (Mc Lennan and Kulesa, 2010) 。目前还不清楚, 在没有VEGF浓度梯度的情况下, VEGF如何控制神经嵴细胞定向迁移。Wiszniak等 (2015) 根据数学模型指出, VEGF信号的浓度因神经嵴细胞的增殖而降低, 可能这些细胞可以通过内吞作用形成VEGF的浓度梯度。领头的细胞能对VEGF响应, 而后面的细胞则对领头细胞产生的某种未知的信号做出二次响应。还有实验证据 (Mc Lennan et al., 2015) 显示, 后面的细胞有些也能够对VEGF响应。并且, 小鼠神经嵴细胞能自己表达VEGF (Wiszniak et al., 2015) 。因此, VEGF对神经嵴细胞产生的趋化性机制还有待证明。
2.1.5、 干细胞因子 (SCF)
SCF能促进神经嵴细胞衍生细胞的分化和迁移。在体外实验中, SCF能维持黑色素细胞前体细胞的增值活性, 加速黑色素细胞的分化并使细胞向SCF高浓度方向定向迁移, 表明SCF可能参与体内神经嵴细胞趋化性引导的机制 (Rovasio et al., 2012) 。但目前尚没有证据显示SCF在体内对神经嵴细胞产生趋化性作用。
2.1.6、 胶质源性神经营养因子 (GDNF)
GDNF在小鼠胚胎中的肠道区域表达并对肠神经嵴细胞产生影响 (Saito et al., 2012) 。体外实验指出, GDNF使肠神经嵴外植体产生趋化性, 使肠神经嵴细胞衍生细胞迁移出外植体。但体内实验发现, 神经嵴细胞不会对GDNF产生趋化性反应 (Saito et al., 2012) 。因此其在体内对神经嵴细胞产生何种影响尚待研究。
2.1.7、 神经营养因子-3 (NT-3)
NT-3参与神经后期发育的多数过程, 包括神经元前体的存活、增殖和分化。有研究表明NT-3在眼部区域的受体Trk B、Trk C和p75在颅神经嵴细胞中也有所表达并且与颅神经嵴细胞涌入听囊的迁移过程有关。此外体外和体内显示, 神经嵴细胞会被高浓度的NT-3吸引, 产生定向迁移 (Zanin et al., 2013) 。
2.2、 神经嵴细胞的趋化性运动机制
在胚胎发育过程和癌症转移过程中, 旁分泌信号可促进细胞趋化性迁移 (Kulesa et al., 2008;Boldajipour et al., 2010;Friedl et al., 2012) 。在体外, 神经嵴细胞也会对基板细胞分泌的SDF-1产生旁分泌趋化性响应 (Theveneau et al., 2013) 。基板是外胚层发育成头部传感器的增厚结构, 两个群体的正常发育需要神经嵴和基板细胞的相互作用 (Steventon et al., 2014) 。通过接触神经嵴细胞, 神经嵴和基板细胞形成了一个基于N-钙粘素粘合的复合体 (Theveneau et al., 2013) 。在体外, 神经嵴细胞会被基板细胞吸引, 但基板细胞会在钙粘素粘合交点和伪足位点上因接触抑制反应被神经嵴细胞所排斥 (Scarpa et al., 2015) 。
因此, 神经嵴细胞因旁分泌信号产生极化并出现伪足→细胞伪足通过SDF-1/CXCR4维持稳定→神经嵴细胞接触基板细胞→神经嵴细胞因接触抑制而去极化并与基板细胞分开→后面的基板细胞簇失去伪足, 导致基板细胞远离神经嵴→接触抑制解除, 神经嵴细胞再次产生极化 (图2) 。这个循环的迁移过程被称为“追逐模型 (Chase and Run) ” (Theveneau et al., 2013) 。神经嵴在旁分泌趋化性作用下追赶基板细胞, 而基板细胞又因迁移接触抑制而远离神经嵴。这个在神经嵴和基板细胞间的双向相互作用高效地调整了两者向侧面和鳍区域的定向迁移。
一些细胞在迁移时能够维持群体的结合, 例如肿瘤 (Hoelzinger et al., 2007) 和盘基网柄菌 (Kay et al., 2008) 。尽管细胞之间的黏着很微弱, 但多数神经嵴细胞也会维持共同迁移而不是单独迁移 (Kulesa and Fraser, 2000;Theveneau and Mayor, 2011) 。在神经嵴定向迁移过程中, 一些旁分泌信号可以维持细胞集群的稳定, 这种迁移的机制被称为“共吸引 (Co-attraction) ” (Carmona-Fontaine et al., 2011) 。神经嵴细胞能分泌补体因子C3a, 并且表达它的受体C3a R。因此, 在富集神经嵴细胞的位置能够发现高浓度的C3a。C3a信号激活Ra C1, 使那些与相邻细胞失去连接的细胞能够随着C3a的浓度梯度迁移回群体, 抵消了迁移过程中, 细胞接触抑制带来的细胞离散现象, 维持细胞集体迁移的群体完整性。因此, 在抑制C3a或使其受体减少之后, 细胞群因接触抑制而离散 (Carmona-Fontaine et al., 2011) 。C3a和C3a R也在非洲爪蟾胚胎的中胚层中被发现 (Mclin et al., 2002) 。在斑马鱼和鸟类胚胎中, 神经嵴细胞的迁移也存在“Co-attraction”, 不过其分子机制还没有探明。
图2 模型Figure 2 The mechanism
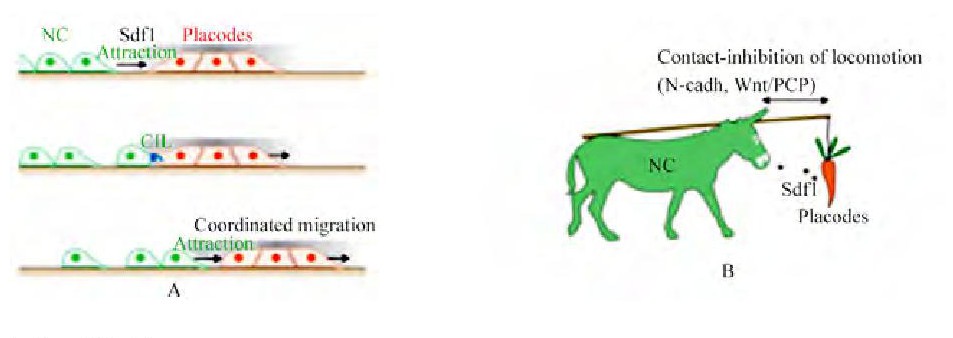
注:A:追逐模型 (Chase and Run) 的机理;B:神经嵴细胞和基板细胞的关系;NC:神经嵴细胞;Sdf1:间质细胞衍生因子;CIL:细胞接触抑制;A的机理:神经嵴细胞跟随基板细胞迁移, 并在接触抑制后分开;B:神经嵴细胞和基板细胞的关系如同驴和胡萝卜 (Theveneau et al., 2013) Note:A:The mechanism of Chase and Run;B:The relationship of neural crest cells and placodal cells just like the donkey and the carrot (Theveneau et al., 2013) ;NC:neural crest;Sdf1:stromal cell-derived factor;CIL:Contact inhibition of locomotion;The mechanism of A:the neural crest cells migration follow the placode cells and leave the placode cell when the CLC become;B:The relationship of neural crest cells and placodal cells just like the donkey and the carrot
2.3、 小结
趋化性作为一种细胞定向迁移的模式, 现在已经被学术界广泛认同。许多信号分子, 如SDF-1和VEGF等都是引起细胞趋化性的趋化因子, 但其浓度梯度尚未在活体胚胎内检测到。许多新发现的机理如“Chase and Run”、“Co-attraction”等也都需要后续试验的进一步证实。
3、 神经嵴细胞的趋电性
现代电生理学的创始人du Bois-Reymond (1843) 详细记录了与神经激发、肌肉收缩和创伤相关的电活动。虽然在神经系统和肌肉运动中, 电信号是公认的概念, 但在创伤修复以及细胞迁移方面, 关于电信号作用的研究相对较少。20年前, 一些研究 (Metcalf et al., 1994;Lowry, 1999) 发现, 在两栖类胚胎发育过程中, 检测到沿着神经嵴由内向外流动的微弱电流, 暗示了神经嵴细胞迁移与电信号的相关性。近年来有研究 (Zhao et al., 2006) 指出, 电信号在指导细胞迁移过程中发挥的作用, 可能比想象中还要重要。因为它在引导伤口愈合细胞迁移中的作用覆盖了其它所有信号。值得注意的是, 与纯粹的电泳不同, 趋电性是活细胞主动响应电信号的过程, 而死去的细胞不会产生趋电性。
3.1、 内源性生理电场
内源性电场 (endogenous electric fields, EFs) 存在于许多生理过程中, 例如组织再生、创伤修复、胚胎发育和肿瘤转移。这是一种跨组织的电势差引起的生理现象, 受到包括Na+、K+、Cl-等带电离子的影响。过去10年的研究 (Nishimura et al., 1996;Fang et al., 1998;Mc Caig et al., 2002;Nuccitelli, 2003;Mc Caig et al., 2005;Levin, 2007) 提供了足够的证据表明, EFs能在伤口愈合中起作用, 指导细胞迁移至目标区域。在两栖类胚胎发育过程中, 外胚层上皮细胞会将Na+等阳离子泵入胚胎内部, 形成EFs (Erickson and Nuccitelli, 1984) 。
3.2、 神经嵴细胞在电场中的响应
许多细胞都对EFs产生趋电性响应并定向迁移至目标区域 (Nuccitelliand Erickson, 1983;Robinson, 1985;Nishimura et al., 1996) 。有研究 (Stump and Robinson, 1983;Gruler and Nuccitelli, 1991;Nishimura et al., 1996) 指出非洲爪蟾神经嵴细胞在10 m V/mm或更大的施加电场中向阴极迁移。并且与神经嵴细胞趋化性不同的是, 在单个细胞和群体细胞中均发现了趋电性。当细胞与其他细胞接触时, 定向迁移的速度通常下降 (Stump and Robinson, 1983) 。也就是说, 细胞的趋电性并不依赖于细胞之间的接触。在低电压下 (<50 m V/mm) , 非洲爪蟾胚胎神经嵴细胞对直流电场的识别和感应通常需要2 h左右的时间。在感应到电场之后开始向阴极迁移, 转换电极后, 细胞能在16~17 min内识别新的电场, 并向阴极迁移。同时还观察到一部分神经嵴细胞会在迁移过程中出现垂直于电场方向的伸长 (Stump and Robinson, 1983;Gruler and Nuccitelli, 1991) 。但是需要注意的是, 上述实验所使用的神经嵴细胞均经过了18 h以上的取材时间, 该细胞可能已经分化为了其他种类的细胞, 如黑色素细胞。
本实验室最近的一项研究 (暂未发表) 采用了时间较短的取材方法 (1~2 h) (Theveneau et al., 2010) , 研究显示在较高电压下 (50~200 m V/mm) , 非洲爪蟾神经嵴细胞在直流电场中迁移至阳极, 细胞对电场的感应时间不超过10 min。并且, 在电极转换后能在5 min内感应并调整迁移方向, 重新向阳极迁移。
上述两种相矛盾的研究暗示, 神经嵴细胞可能在分化之后会产生趋电性的改变。但需要警觉的是, 活的神经嵴细胞膜表面是带负电的 (Stump and Robinson, 1983) , 这提示了我们在较高电压下的神经嵴细胞迁移可能存在电泳效应, 至少存在一部分。
3.3、 神经嵴细胞趋电性机理
目前, 有一些重要的电信号介导分子已经被鉴定出来, 例如EGFR (Zhao et al., 1999) 、Rac1 (Pullar and Isseroff, 2005) 、V-ATPase H+pump (Adams et al., 2007) 和PI3 kinase/Pten (Phosphoinositide 3-kinases/phosphatase and tensin homolog) (Zhao et al., 2006) 。这些电信号的传导受体或许能帮助我们理解细胞是如何主动响应电信号的。下面介绍两种分子受体的作用机理, 作为神经嵴细胞趋电性迁移的参考。
3.3.1、 磷酸肌醇-3激酶/磷酸酶和张力蛋白同源物 (PI3 kinase/Pten)
PI3kinase和Pten是引起细胞极化的重要分子。当细胞检测到迁移信号 (趋化因子) 时, PI3kinase会移动到细胞的前沿, 而Pten则会移动到细胞的后沿, 使细胞产生极化 (Zhao et al., 2006;Huttenlocher, 2007) 。嗜中性粒细胞和角质形成层细胞中的PI3kinase信号通路可以被EFs激活, 这种激活非常迅速。表达Akt-GFP的细胞在EFs中能显示出细胞在迁移的方向上被极化, 表明PI3kinase在阴极面活化, 细胞前沿产生伪足;当EFs两极反转时, Akt-GFP分布到了新的阴极表面。这种现象说明了PI3kinase能够被EFs激活并影响细胞极化, 使细胞能在迁移方向上产生伪足, 并沿EFs方向定向迁移 (Zhao et al., 2006;Huttenlocher, 2007) 。当使用药物消除PI3kinase的影响后, 细胞的趋电迁移现象消失。而在Pten基因被删除后的角质形成层细胞显示出Akt磷酸化增强的现象 (Zhao et al., 2006) 。
3.3.2、 表皮生长因子受体 (EGFR)
角膜和皮肤上皮细胞在EFs中的极化受到EGFR信号诱导, 使用EGF-FITC (EGF fluorescein conjugate) 可以观察到EGFR分布在细胞的阴极一侧 (Zhao et al., 1999) 。ERK1/2和肌动蛋白丝也分布到阴极一侧 (Fang et al., 1999) 。电势差可能激活阴极面对侧的膜受体, 然后通过细胞内的下游信号通路诱导极化, 最终导致极化的肌动蛋白丝在阴极聚合并促使细胞定向迁移 (Fang et al., 1999;Huttenlocher, 2007) 。
3.4、 小结
细胞趋电性是指导细胞定向迁移的重要特性, 在伤口愈合和胚胎发育过程中具有很重要的意义。近年来, 关于细胞趋电性的研究开始得到重视, 越来越多的趋电基因和信号分子被发现, 如PI3 kinase/Pten、EGFR等。然而, 目前对趋电性的研究仍处在起步阶段, 许多和趋电性相关的信号通路尚待发现, 也还有很多机理和假说需要实验研究来证明。
4、 讨论与展望
作为在胚胎发育过程中机动性最好的细胞群体, 神经嵴细胞一直都是非常理想的研究细胞定向迁移的模型。对神经嵴细胞迁移的研究, 在揭示胚胎发育机理、创伤修复和肿瘤治疗等领域都有着深远的意义。而研究趋化性和趋电性这两种细胞在迁移过程中的特性, 能帮助我们更好的理解神经嵴细胞是如何在胚胎内完成如此复杂的迁移和分化的。需要指出的是, 趋化性和趋电性的作用不应该是对立的, 而是相互补充和支持的。同时, 趋化性和趋电性又是有所区别的。不同的信号分子能指导不同的神经嵴细胞亚型迁移到不同的区域, 而这些信号分子所产生的电势差又能在同一路径上引导不同神经嵴细胞亚型向一个方向迁移。
无论是细胞趋化性还是趋电性, 都还有太多的问题需要解决。诸如趋化因子在活体内的浓度梯度, 电信号的识别和感应等等。这些研究不应该局限于生物学方面, 跨学科的研究很有可能帮助我们揭示很多一直被忽略的问题。但无论如何, 趋化性和趋电性都是非常有魅力的两种细胞固有属性。随着显微成像技术和分子生物学技术的发展, 越来越多的细胞趋化性和趋电性机理将会被发现并证明, 神经嵴细胞迁移的研究也将会有更好前景。
参考文献:
[1]Abu-Issa R., Smyth G., Smoak I., Yamamura K.I., and Meyers E.N., 2002, Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse, Development, 129 (19) :4613-4625
[2]Adams D.S., Masi A., and Levin M., 2007, H+pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration, Development, 134 (7) :1323-1335
[3]Alfandari D., Cousin H., Gaultier A., Hoffstrom B.G., and De Simone D.W., 2003, Integrinα5β1 supports the migration of Xenopus cranial neural crest on fibronectin, Developmental Biology, 260 (2) :449-464
[4]Barriga E.H., Maxwell P.H., Reyes A.E., and Mayor R., 2013, The hypoxia factor Hif-1αcontrols neural crest chemotaxis and epithelial to mesenchymal transition, J.Cell Biol., 201 (5) :759-776
[5]Barriga E.H., Trainor P.A., Bronner M., and Mayor R., 2015, Animal models for studying neural crest development:is the mouse different?Development, 142 (9) :1555-1560
[6]Belmadani A., Jung H., Ren D., and Miller R.J., 2009, The chemokine SDF-1/CXCL12 regulates the migration of melanocyte progenitors in mouse hair follicles, Differentiation, 77 (4) :395-411
[7]Boldajipour B., Mahabaleshwar H., Kardash E., Reichman-Fried M., Blaser Heiko., Minina S., Wilson D., Xu Q., and Raz E., 2008, Control of chemokine-guided cell migration by ligand sequestration, Cell, 132 (3) :463-473
[8]Braun M., Wunderlin M., Spieth K., Knoochel W., Gierschik P., and Moepps B., 2002, Xenopus laevis stromal cell-derived factor 1:conservation of structure and function during vertebrate development, The Journal of Immunology, 168 (5) :2340-2347
[9]Butler S.J., and Dodd J., 2003, A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons, Neuron, 38 (3) :389-401、
[10]Cai D., and Montell D.J., 2014, Diverse and dynamic sources and sinks in gradient formation and directed migration, Current Opinion Cell Biology, 30:91-98
[11]Carmona-Fontaine C., Matthews H., and Mayor R., 2014, Directional cell migration in vivo, Cell Adhesion and Migration, 2 (4) :240-242
[12]Carmona-Fontaine C., Theveneau E., Tzekou A., Tada M., Woods M., Page K.M., Parsons M., Lambris J.D., and Mayor R., 2011, Complement fragment C3a controls mutual cell attraction during collective cell migration, Developmental Cell, 21 (6) :1026-1037
[13]Cavanaugh A.M., Huang J., and Chen J.N., 2015, Two developmentally distinct populations of neural crest cells contribute to the zebrafish heart, Developmental Biology, 404 (2) :103-112
[14]Cebra-Thomas J.A., Terrell A., Branyan K., Shah S., Rice R., Gyi L., Yin M., Hu Y., Mangat G., Simonet J., Betters E., and Gilbert S.F., 2013, Late-emigrating trunk neural crest cells in turtle embryos generate an osteogenic ectomesenchyme in the plastron, Developmental Dynamics, 242 (11) :1223-1235
[15]Charras G., and Sahai E., 2014, Physical influences of the extracellular environment on cell migration, Nature Reviews Molecular Cell Biology, 15 (12) :813-824
[16]Das T., Safferling K., Rausch S., Grabe N., Boehm H., and Spatz J.P., 2015, A molecular mechanotransduction pathway regulates collective migration of epithelial cells, Nature Cell Biology, 17 (3) :276-287
[17]Ding X., Zhao Z., Duan W., Wang S., Jin X., Xiang L., and Jin X., 2013, Expression patterns of CXCR4 in different colon tissue segments of patients with Hirschsprung's disease, Experimental Molecular Pathology, 95 (1) :111-116
[18]du Bois-Reymond E., 1843, Vorlufiger Abriss einer Untersuchungüueber den sogenannten Froschstrom undüueber die elektromotorischen Fische, Annalen der Physik, 134 (1) :1-30
[19]Duband J.L., Dady A., and Fleury V., 2015, Resolving time and space constraints during neural crest formation and delamination, Current Topics Developmental Biology, 111:27-67
[20]Dupin E., Creuzet S., and Le Douarin N.M., 2006, The contribution of the neural crest to the vertebrate body, In:Saint-Jeannet J.P. (ed.) , Neural crest induction and differentiation, landes bioscience and springer science+business media, London, UK, pp.96-119
[21]Engelmann T.T.W., ed., 1882, Ueber assimilation von haematococcus, Bot Ztg, London, UK, pp.419-426
[22]Erickson C.A., and Nuccitelli R., 1984, Embryonic fibroblast motility and orientation can be influenced by physiological electric fields, The Journal of cell biology, 98 (1) :296-307
[23]Escot S., Blavet C., Hartle S., Duband J.L., and Fournier-Thibault C., 2013, Misregulation of SDF1-CXCR4 signaling impairs early cardiac neural crest cell migration leading to conotruncal defects, Circulation Research, 113 (5) :505-516
[24]Fang K.S., Ionides E., Oster G., Nuccitelli R., and Isseroff R.R., 1999, Epidermal growth factor receptor relocalization and kinase activity are necessary for directional migration of keratinocytes in DC electric fields, Journal of Cell Science, 112 (12) :1967-1978
[25]Fang K.S., Farboud B., Nuccitelli R., and Isseroff R.R., 1998, Migration of human keratinocytes in electric fields requires growth factors and extracellular calcium, Journal of Investigative Dermatology, 111 (5) :751-756
[26]Frank D.U., Fotheringham L.K., Brewer J.A., Muglia L.J., Tristani-Firouzi M., Capecchi M.R., and Moon A.M., 2002, An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome, Development, 129 (19) :4591-4603
[27]Friedl P., and Gilmour D., 2009, Collective cell migration in morphogenesis, regeneration and cancer, Nature Reviews Molecular Cell Biology, 10 (7) :445-457
[28]Friedl P., Sahai E., Weiss S., and Yamada K.M., 2012, New dimensions in cell migration, Nature Reviews Molecular Cell Biology, 13 (11) :743-747
[29]Gammill L.S., and Roffers-Agarwal J., 2010, Division of labor during trunk neural crest development, Developmental Biology, 344 (2) :555-565
[30]Gilbert S.F., Bender G., Betters E., Yin M., and Cebra-Thomas J.A., 2007, The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell, Integrative Comparative Biology, 47 (3) :401-408
[31]Goto A., Sumiyama K., Kamioka Y., Nakasyo E., Ito K., Iwasaki M., Enomoto H., and Matsuda M., 2013, GDNF and endothelin 3 regulate migration of enteric neural crest-derived cells via protein kinase A and Rac1, The Journal Neuroscience, 33 (11) :4901-4912
[32]Gruler H., and Nuccitelli R., 1991, Neural crest cell galvanotaxis:new data and a novel approach to the analysis of both galvanotaxis and chemotaxis, Cell Motility and The Cytoskeleton, 19 (2) :121-133
[33]He F., and Soriano P., 2013, A critical role for PDGFRαsignaling in medial nasal process development, PLo S Genetics, 9 (9) :e1003851
[34]High F.A., Jain R., Stoller J.Z., Antonucci N.B., Lu M.M., Loomes K.M., Kaestner K.H., Pear W.S., and Epstein J.A., 2009, Murine Jagged1/Notch signaling in the second heart field orchestrates Fgf8 expression and tissue-tissue interactions during outflow tract development, The Journal of Clinical Investigation, 119 (7) :1986-1996
[35]Hoelzinger D.B., Demuth T., and Berens M.E., 2007, Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment, Journal National Cancer Institute, 99 (21) :1583-1593
[36]Huttenlocher A., 2007, Wound healing with electric potential, The New England Journal of Medicine, 356 (3) :303
[37]Kasemeier-Kulesa J.C., Mc Lennan R., Romine M.H., Kulesa P.M., and Lefcort F., 2010, CXCR4 controls ventral migration of sympathetic precursor cells, Journal Neuroscience, 30 (39) :13078-13088
[38]Kawakami M., Umeda M., Nakagata N., Takeo T., and Yamamura K.I., 2011, Novel migrating mouse neural crest cell assay system utilizing P0-Cre/EGFP fluorescent time-lapse imaging, BMC Developmental Biology, 11 (1) :68
[39]Kay R.R., Langridge P., Traynor D., and Hoeller O., 2008, Changing directions in the study of chemotaxis, Nature Reviews Molecular Cell Biology, 9 (6) :455-463
[40]Kelsh R.N., Harris M.L., Colanesi S., and Erickson C.A., 2009, Stripes and belly-spots-a review of pigment cell morphogenesis in vertebrates, Seminars in Cell and Developmental Biology, 20 (1) :90-104
[41]Kirby M.L., and Hutson M.R., 2014, Factors controlling cardiac neural crest cell migration, Cell Adhesion and Migration, 4 (4) :609-621
[42]Krause M., and Gautreau A., 2014, Steering cell migration:lamellipodium dynamics and the regulation of directional persistence, Nat Reviews Molecular Cell Biology, 15 (9) :577-590
[43]Kulesa P.M., and Mc Lennan R., 2015, Neural crest migration:trailblazing ahead, F1000 Prime Reports, 7:02
[44]Kulesa P.M., Bailey C.M., Kasemeier-Kulesa J.C., and Mc Lennan R., 2010, Cranial neural crest migration:new rules for an old road, Developmental Biology, 344 (2) :543-554
[45]Kulesa P., Ellies D.L., and Trainor P.A., 2004, Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis, Developmental Dynamics, 229 (1) :14-29
[46]Kulesa P.M., and Fraser S.E., 2000, In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches, Development, 127 (6) :1161-1172
[47]Kuo B.R., and Erickson C.A., 2014, Regional differences in neural crest morphogenesis, Cell Adhesion and Migration, 4 (4) :567-585
[48]Kuriyama S., Theveneau E., Benedetto A., Parsons M., Tanaka M., Charras G., Kabla A., and Mayor R., 2014, In vivo collective cell migration requires an LPAR2-dependent increase in tissue fluidity, Journal Cell Biology, 206 (1) :113-127
[49]Levin M., 2007, Large-scale biophysics:ion flows and regeneration, Trends Cell Biology, 17 (6) :261-70
[50]Loring J.F., and Erickson C.A., 1987, Neural crest cell migratory pathways in the trunk of the chick embryo, Developmental biology, 121 (1) :220-236
[51]Lowry C., 1999, How electric fields mold the embryo's growth pattern and shape, 21st Century Science Technology, pp.56-70
[52]Macatee T.L., Hammond B.P., Arenkiel B.R., Francis L., Frank D.U., and Moon A.M., 2003, Ablation of specific expression domains reveals discrete functions of ectoderm-and endoderm-derived FGF8 during cardiovascular and pharyngeal development, Development, 130 (25) :6361-6374
[53]Matthews H.K., Marchant L., Carmona-Fontaine C., Kuriyama S., Larrain J., Holt M.R., Parsons M., and Mayor R., 2008, Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/Rho A, Development, 135 (10) :1771-1780
[54]Mc Caig C.D., Rajnicek A.M., Song B., and Zhao M., 2002, Has electrical growth cone guidance found its potential?Trends in Neurosciences, 25 (7) :354-359
[55]Mc Caig C.D., Rajnicek A.M., Song B., and Zhao M., 2005, Controlling cell behavior electrically:current views and future potential, Physiological Reviews, 85 (3) :943-978
[56]Mc Lennan R., and Kulesa P.M., 2007, In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick, Developmental Biology, 301 (1) :227-239
[57]Mc Lennan R., and Kulesa P.M., 2010, Neuropilin-1 interacts with the second branchial arch microenvironment to mediate chick neural crest cell dynamics, Developmental Dynamics, 239 (6) :1664-1673
[58]Mc Lennan R., Schumacher L.J., Morrison J.A., Teddy J.M., Ridenour D.A., Box A.C., Semerad C.L., Li H., Mc Dowell W., Kay D., Maini P.K., Baker R.E., and Kulesa P.M., 2015, VEGF signals induce trailblazer cell identity that drives neural crest migration, Developmental Biology, 407 (1) :12-25
[59]Mc Lennan R., Teddy J.M., Kasemeier-Kulesa J.C., Romine M.H., and Kulesa P.M., 2010, Vascular endothelial growth factor (VEGF) regulates cranial neural crest migration in vivo, Developmengtal Biollgy, 339 (1) :114-125
[60]Mclin A., Hu Cheng.H., Shah R., and Jamrich M., 2002, Expression of complement components coincides with early patterning and organogenesis in Xenopus laevis, International Journal of Developmental Biology, 52 (8) :1123-1133
[61]Metcalf M.E., Shi R., and Borgens R.B., 1994, Endogenous ionic currents and voltages in amphibian embryos, Journal of Experimental Zoology Part A:Ecological Genetics and Physiology, 268 (4) :307-322
[62]Morrison-Graham K., Schatteman G.C., Bork T., Bowen-Pope D.F., and Weston J.A., 1992, A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells, Development, 115 (1) :133-142
[63]Nieto M.A., 2013, Epithelial plasticity:a common theme in embryonic and cancer cells, Science, 342 (6159) :708
[64]Nikitina N., Sauka-Spengler T., and Bronner-Fraser M., 2008, Dissecting early regulatory relationships in the lamprey neural crest gene network, Proceedings of the The National A-cademy of Sciences of the United States of America, 105 (51) :20083-20088
[65]Nishimura K.Y., Isseroff R.R., and Nuccitelli R., 1996, Human keratinocytes migrate to the negative pole in direct current electric fields comparable to those measured in mammalian wounds, Journal of Cell Science, 109 (1) :199-207
[66]Nuccitelli R., 2003, A Role for Endogenous Electric Fields in Wound Healing, Current Topics in Developmental Biology, 58 (2) :1-26
[67]Nuccitelli R., and Erickson C.A., 1983, Embryonic cell motility can be guided by physiological electric fields, Experimental Cell Research, 147 (1) :195-201
[68]Ota K.G., Kuraku S., and Kuratani S., 2007, Hagfish embryology with reference to the evolution of the neural crest, Nature, 446 (7136) :672-675
[69]Pfeffer W.F.P., ed, 1884, Locomotorische richtungsbewegungen durch chemische reize, Unters Bot Inst, Tübingen, UK, pp.363-482
[70]Pullar C.E., and Isseroff R.R., 2005, Cyclic AMP mediates keratinocyte directional migration in an electric field, Journal of Cell Science, 118 (9) :2023-2034
[71]Rezzoug F., Seelan R.S., Bhattacherjee V., Greene R.M., and Pisano M.M., 2011, Chemokine-mediated migration of mesencephalic neural crest cells, Cytokine, 56 (3) :760-768
[72]Riahi R., Sun J., Wang S., Long M., Zhang D.D., and Wong P.K., 2015, Notch1-Dll4 signalling and mechanical force regulate leader cell formation during collective cell migration, Nature Communications, 6:6556
[73]Richarte A.M., Mead H.B., and Tallquist M.D., 2007, Cooperation between the PDGF receptors in cardiac neural crest cell migration, Developmental Biology, 306 (2) :785-796
[74]Ricoult S.G., Kennedy T.E., and Juncker D., 2015, Substrate-bound protein gradients to study haptotaxis, Fronters in Bioengineering Biotechnology, 3:40
[75]Robinson K.R., 1985, The responses of cells to electrical fields:a review, Journal of Cell Biology, 101 (6) :2023-2027
[76]Roca-Cusachs P., Sunyer R., and Trepat X., 2013, Mechanical guidance of cell migration:lessons from chemotaxis, Current Opinion Cell Biology, 25 (5) :543-549
[77]Rogers C.D., Jayasena C.S., Nie S., and Bronner M.E., 2012, Neural crest specification:tissues, signals, and transcription factors, Wiley Interdiscip, Wiley Interdisciplinary Reviews Developmental Biology, 1 (1) :52-68
[78]Roussos E.T., Condeelis J.S., and Patsialou A., 2011, Chemotaxis in cancer, Nature Reviews Cancer, 11 (8) :573-587
[79]Rovasio R.A., Faas L., and Battiato N.L., 2012, Insights into stem cell factor chemotactic guidance of neural crest cells revealed by a real-time directionality-based assay, European Journal Cell Biology, 91 (5) :375-390
[80]Roycroft A., and Mayor R., 2016, Molecular basis of contact inhibition of locomotion, Cellular Molecular Life Sciences, 73 (6) :1119-1130
[81]Saika S., Saika S., Liu C.Y., Azhar M., Sanford L.P., Doetschman T., Gendron R.L., Kao C.W.C., and Kao W.W.Y., 2001, TGFbeta2 in corneal morphogenesis during mouse embryonic development, Developmental Biology, 240 (2) :419-432
[82]Saito D., Takase Y., Murai H., and Takahashi Y., 2012, The dorsal aorta initiates a molecular cascade that instructs sympatho-adrenal specification, Science, 336 (6088) :1578-1581
[83]Sato A., Scholl A.M., Kuhn E.N., Stadt H.A., Decker J.R., Pegram K., Hutson M.R., and Kirby M.L., 2011, FGF8 signaling is chemotactic for cardiac neural crest cells, Developmental Biology, 354 (1) :18-30
[84]Scarpa E., Szabo A., Bibonne A., Theveneau E., Parsons M., and Mayor R., 2015, Cadherin switch during EMT in neural crest cells leads to contact inhibition of locomotion via repolarization of forces, Developmental Cell, 34 (4) :421-434
[85]Shellard A., and Mayor R., 2016, Chemotaxis during neural crest migration, Seminars in Cell and Developmental Biology, 55:111-118
[86]Steventon B., Mayor R., and Streit A., 2014, Neural crest and placode interaction during the development of the cranial sensory system, Developmental Biology, 389 (1) :28-38
[87]Stump R.F., and Robinson K.R., 1983, Xenopus neural crest cell migration in an applied electrical field, Journal of Cell Biology, 97 (4) :1226-1233
[88]Tallquist M.D., Weismann K.E., Hellstrom M., and Soriano P., 2000, Early myotome specification regulates PDGFA expression and axial skeleton development, Development, 127 (23) :5059-5070
[89]Teddy J.M., and Kulesa P.M., 2004, In vivo evidence for shortand long-range cell communication in cranial neural crest cells, Development, 131 (24) :6141-6151
[90]Theveneau E., and Mayor R., 2011, Can mesenchymal cells undergo collective cell migration?The case of the neural crest, Cell Adhesion and Migration, 5 (6) :490-498
[91]T heveneau E., and Mayor R., 2012, Neural crest delamination and migration:from epithelium-to-mesenchyme transition to collective cell migration, Developmental Biology, 366 (1) :34-54
[92]Theveneau E., Marchant L., Kuriyama S., Gull M., Moepps B., Parsons M., and Mayor R., 2010, Collective chemotaxis requires contact-dependent cell polarity, Developmental Cell, 19 (1) :39-53
[93]Theveneau E., Steventon B., Scarpa E., Garcia S., Trepat X., Streit A., and Mayor R., 2013, Chase-and-run between adjacent cell populations promotes directional collective migration, Nature Cell Biology, 15 (7) :763-772
[94]Thiery J.P., Acloque H., Huang R.Y., and Nieto M.A., 2009, Epithelial-mesenchymal transitions in development and disease, Cell, 139 (5) :871-890
[95]Toyofuku T., Yoshida J., Sugimoto T., Yamamoto M., Makino N., Takamatsu H., Takegahara N., Suto F., Hori M., Fujisawa H., Kumanogoh A., and Kikutani H., 2008, Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells, Developmental Biology, 321 (1) :251-262
[96]Walshe J., and Mason I., 2003, Unique and combinatorial functions of Fgf3 and Fgf8 during zebrafish forebrain development, Development, 130 (18) :4337-4349
[97]Wiszniak S., Mackenzie F.E., Anderson P., Kabbara S., Ruhrberg C., and Schwarz Q., 2015, Neural crest cell-derived VEGFpromotes embryonic jaw extension, Proceedings of the National Academy of Sciences, 112 (19) :6086-6091
[98]Zanin J.P., Battiato N.L., and Rovasio R.A., 2013, Neurotrophic factor NT-3 displays a non-canonical cell guidance signaling function for cephalic neural crest cells, European Journal of Cell Biology, 92 (8-9) :264-79
[99]Zhao M., Dick A., Forrester J.V., and Mc Caig C.D., 1999, Electric field-directed cell motility involves up-regulated expression and asymmetric redistribution of the epidermal growth factor receptors and is enhanced by fibronectin and laminin, Molecular Biology of the Cell, 10 (4) :1259-1276
[100]Zhao M., Song B., Pu J., Wada T., Reid B., Tai G., Wang F., Guo A., Walczysko P., Gu Y., Sasaki T., Suzuki A., Forrester J.V., Bourne H.R., Devreotes P.N., Mc Caig C.D., and Penninger J.M., 2006, Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN, Nature, 442:457-460
注释:
13799-3809 (蒋锐达, 赵敏, 赵三军, 施利民, 高润池, 王晓燕, 2018, 胚胎发育中神经嵴细胞迁移机制的研究进展, 基因组学与应用生物学, 37 (9) :3799-3809)