����ժ Ҫ�������ؿ�ת��Ϊ���ָ߸���ֵ��Ʒ��ϵ�л�ѧƷ�������������Լ��ؼ��併�����N-��������������ת���Ʊ�ƽ̨������3-��������-5-������ૺ���������������Ȼ�ѧƷ�������о���չ������3-��������-5-���������Ϊƽ̨�������һ��ת��Ϊ��������������˸�����������Щ������о����������չ�������ڴٽ�����ת��������ѧƷ����ķ�չ��
�����ؼ��ʣ�������; 3-��������-5-�������; N-��������������; ������ѧƷ;
����Abstract����Chitin can be converted into a variety of high value-added products and chemicals. The latest research progress in the conversion of chitin and its degradation product of N-acetyl glucosamine to nitrogen-containing chemicals such as 3-acetamido-5-acetylfuran and other N-containing compounds is reviewed. The further conversion of 3-acetamido-5-acetylfuran as a platform compound to other compounds is also summarizes. The research directions in these fields are prospected so as to promote the development of chitin conversion into nitrogen-containing chemicals.
����Keyword����chitin; 3-acetamido-5-acetylfuran; N-acetyl-D-glucosamine; nitrogen-containingchemicals;
������Դ����ѧƷ�Ͳ�������ᾭ�÷�չ����Ҫ���ʻ�����������������ʯ��Դ�Ĺ㷺�������õ�����Դ�ݽߣ�ͬʱ�����˻�����Ⱦ�����⣬��������������Ĺ㷺��ע����������Դ�Ŀ��������ܵ�Խ��Խ��Ĺ�ע[1]����������һ����Ȼ����Դ�ḻ����ɫ��������Դ�����Ʊ���Դ����ѧƷ�Ͳ��ϵ����������̼ԭ��[2,3,4]��ͨ������/��ѧ���ƿɽ���ת��Ϊ�������P������Ʒ[5,6]�������������ﻯ����Ʒ��������ԭ��ͨ���ɷ�Ϊ���֣������ǻ������ۻ���ľ����ά������Դ[7,8]��Ŀǰ����������������ȼ�Ϻͻ�ѧƷ�õ��˹㷺�о�����ȡ���˺ܴ��չ[9,10]����ʹ��ľ����ά���Ʊ������Ҵ�[11]������[12]��1,5-�������1,6-������[13]��Һ��ȼ�Ϻ;�ϸ������Ʒ������Щ���̴���������ں�C��H��Oԭ�ӵ������ǻ���������Դ�����������о��߶���������ԭ�ӵ�������ת��Ϊ��ѧ���ʵ���Ȥ����Ũ��[14,15]��
�������أ��ֳ�Ϊ�����ʡ����ʣ���N-�������������ǣ�N-acetyl-D-glucosamine��GlcNAc��ͨ��β-1,4���ռ����Ӷ���[16]���ǿ�����̼�Ǽ���Դ�в�����������ά�ص�����ۺ���[17]��ͬʱҲ����Ȼ������Ψһ�ĺ������ǡ����ذ����������еIJ����Ϊα��β��γ���ֽᾧͬ��������[18,19]��α�����������ط���ƽ��ͨ������������ι̽����һ����������и߸��Խṹ��β������ͬ��ƽ�У�û�з���������������ڷ��Ӽ�����������α�;��и��ٵĸ��Խṹ��γ�������ƽ�кͷ���ƽ�����ֽṹ[20]���й������ڶ࣬�������������ڴ�����ҵ�ӹ�����Ʒ����Ϻз�ǡ�����ǡ������ж����м���[21]�����⣬���ػ��㷺��������Ȼ������������Լ�ijЩ���ࡢ����ȵ�ϸ�����У�ÿ����Ȼ���ɵļ��ظߴ�1.0×10 10��[22]����Щ�ḻ����ԴΪת���Ʊ������������ṩ�˷ḻԭ�ϡ��о���Ա������Ҫ���о��ص�����Լ���Ϊԭ���Ʊ����ػ�������ϡ��Ǿ��Ǽ��������ĸ��ֹ��ǡ�GlcNAc�ȸ߸���ֵ��Ʒ[23]����������ڼ��صľ���Դ��������ռ������С��Ŀǰ���ϳɺ�����ѧƷͨ����Ҫ���º�ѹ�İ��ϳɷ������ֻ�ѧת�����̣����к��ܸߡ��������ʵ͡������������⡣���ڼ����к�����Ȼ�ĵ���������ת���Ʊ�������ѧƷ�����˹㷺��ע[24]���Լ��ؼ��䵥��N-��������������ת���Ʊ������������д����о��Σ�δʵ�ֹ�ҵ�������������о��Լ��ٻ�����Ⱦ�������������Դ�������ʡ��ٽ���ɫѭ�����÷�չ������Ҫ����[25]��

������������ת�����÷���������������Ʊ���ֵ����Ʒ���棬�Ժ�����ѧƷ�Ʊ�Ӧ�õIJ������١����ĶԼ���������ת���Ʊ�������ѧƷ���о���չ�������������ص�����˼����Ʊ�3-��������-5-������ૣ�3-acetamido-5-acetylfuran��3A5AF��������������ѧƷ���о���״����3A5AFΪƽ̨������ת��Ϊ������ѧƷ�����½�չ������δ����������о����������չ�����Դٽ�����ת��Ϊ������ѧƷ�ķ�չ���ƶ����ܡ������Ϳɳ����ķ��������Ŀǰ���ֺ�����ѧƷ������ģʽ��
����1�����ؿ��Ʊ�ת��Ϊ���ֻ�����
�����Լ��ؼ�����ǡ�����Ϊԭ��ת��Ϊ����������ı����ܶ࣬��ͼ1��ʾ��
����ͼ1 ���ؿ��Ʊ��Ļ�ѧƷ[26]
![ͼ1 ���ؿ��Ʊ��Ļ�ѧƷ[26]](http://www.xueshut.com/uploads/allimg/200706/36-200F610560C26.jpg)
����Fig. 1 Chemicalsprepared from chitin[26]
������Щ������ɷ�Ϊ���࣬һ���Dz������Ļ����3~7������Ҫ�����ᣨ4�������ᣨ5�����������ᣨ6��������������ͪ��7���Լ�5-�Ǽ���ȩ��3��[27]�ȣ���Щ��������Դ������������Ķ���/�����Ʊ��õ�����һ���Ǻ����Ļ����8~16������Ҫ����3A5AF��9����N-���������Ҵ�����10������ण�11����������12������ຣ�13�������һ�-2-����-2-������������գ�15�������Ҵ�����16���ȡ�GlcNAc��14����һ�ְ����ǣ��Ǽ��ؽ���õ��ĵ���[16]������ڼ��ؽ����ƻ��Ľṹ����GlcNAcΪԭ����һ�������ƣ��������ܽ⡢Ŀ���������ʽϸߵȡ�GlcNAc���Ʊ����ָ߸���ֵ�ĺ��������ͼ2������3A5AF��9����ɫԭ���17��18����2-��������-3,6-��ˮ-2-����-D-��������ǣ�19����2-��������-3,6-��ˮ-2-����-D-��¶��ǣ�20���ȣ���Щ�������нϺõ�������ԣ�����Ϊ�����Ե�ʳƷ���Ӽ�[28]�������Ʊ������������������Լ�����������Ⱦ�ͼ������Ⱦ��ҩ��[29]��
����ͼ2 GlcNAc��ת���Ʊ��ĸ��ֺ���������
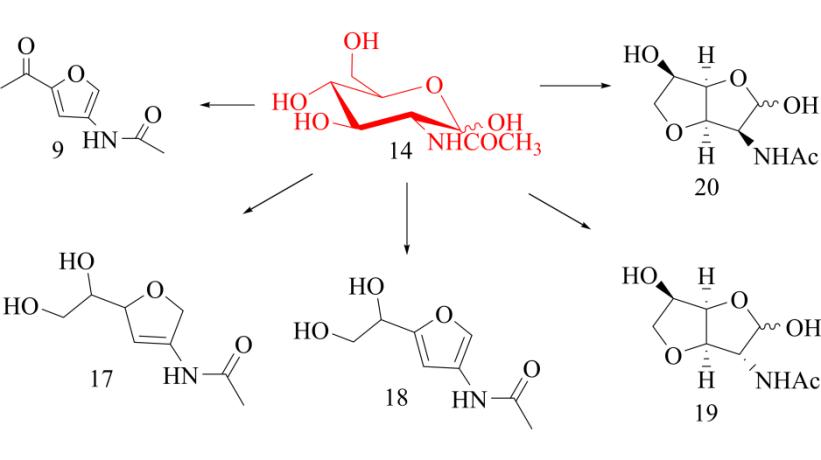
����Fig.2 DifferentN-containing compounds prepared GlcNAc
��������ձ�����ڶ��־���������Ե���Ȼ��Ʒ�ͻ������е���Ԫ�ӻ���ĺϳ��ǵ����л���ѧ�о���һ����Ҫ����[30]���ڼ��غ�GlcNAc���������У�3A5AF�������о��ߵĹ㷺��ע��3A5AF��һ����Ҫ�ĺ���ૻ�����������˼����д��ڵı������ﵪԪ�أ�����Ҫ�������ƽ̨���������Ϊ������Ҫ��������м���Ͷ���ҩ����ӵ�ǰ�壬�翹��ProximicinA���������HyrtioseragamineA/B�Լ�Pyrrolosine��[31]��������������Դ�Թ������Ŀ���������������[32]��
�������⣬������Ϊһ����GlcNAc�ۺ϶��ɵ���Ȼ���ǣ����Ʊ�3A5AF��������Ȼԭ�ϡ������������ⷽ����о�ȡ���˺ܴ�Ľ�չ��
����2N-��������������ת���Ʊ�3A5AF�о���չ
������1984����н�������ת����3A5AF�ı�����FRANICH��[33]�����Ƚ�ķ�ʽ����400 ���½�GlcNAc�����Ƚ⣬ͨ��GC-MS��ⷢ����2%��3A5AF���ɡ�����һ���о���[34]����GlcNAc����ˮ��������ƺ�ʯӢɰ�����200�����ԡ�·�Ӧ30min�����3A5AF���������ʽ���0.04%��OMARI��[14]�������ȵ�220��������£���B��OH��3��Ϊ����������NaCl����N,N-������������N,N-Dimethylacetamide��DMA��Ϊ�ܼ���Ӧ15min��3A5AF�IJ��ʴﵽ��58%��FRANCESCA��[35]�״β�������Һ����Ϊ�÷�Ӧ���ܼ���������Һ��1-����-3-���������Σ�[BMIM]Cl���У�������������£�3A5AF�IJ��ʿ��Դﵽ25.5%���о���������Һ���е������Ӷ�3A5AF�������нϴ�Ӱ�죬����1-ϩ����-3-���������Ρ�1-����-3-����������εȲ��������ӵ�����Һ���У�ֻ��������3A5AF�����Ľ�һ��̽������������Ϊ�����������������������������ӣ����3A5AF������Ҳ��֮���ӣ�����������ﵽĦ������200%ʱ��3A5AF��������ߴﵽ��60%����Ҳ��Ŀǰ������������ʡ��������о������������Լ��������ڷ�Ӧ�����˺ܴ����á�WANG��[36]�������о�������������Һ��[Gly]Cl��200���·�Ӧ10min�����Ի������Ϊ43.22%��3A5AF����һ����÷�Ӧ��ϵ������CaCl2������ʹ3A5AF��������ߵ�52.61%��ѡ���Ըߴ�83.3%�����о�δ�ڷ�ӦҺ�����������Ӷ�ʹ3A5AF��ýϸ����ʣ����������ӵĴ��ڶԲ����ѡ���Խ�Ϊ��Ҫ����1�ܽ���ĿǰGlcNAcת��Ϊ3A5AF�ľ��弼����������Ӧ�����
�������о�GlcNAcת��Ϊ3A5AF��ͬʱ��Ҳ�о����䷴Ӧ������GlcNAcת��Ϊ3A5AF��Ӧ�Ŀ���·����ͼ3��ʾ����������ǻ�ͨ�������õ�����ȩ�ǣ���ξ����ǻ����˹������ջ��γ�����Ԫ��૽ṹ��ͨ��ͪ-ϩ�������칹������ѳ��������ӵ�ˮ�õ�Ŀ�����3A5AF[35]��
����ͼ3 GlcNAc�Ʊ�3A5AF���ܵķ�Ӧ����[35]
![ͼ3 GlcNAc�Ʊ�3A5AF���ܵķ�Ӧ����[35]](http://www.xueshut.com/uploads/allimg/200706/36-200F61055221A.jpg)
����Fig.3Possiblereaction process of conversionof GlcNAc to 3A5AF[35]
������1GlcNAcת��Ϊ3A5AF�о�����ܽ�
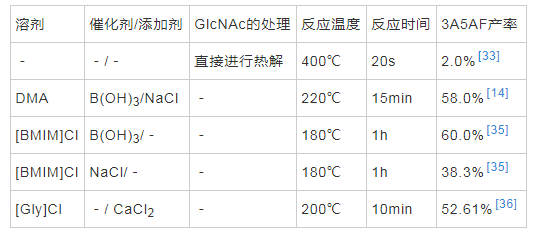
����Ŀǰ����GlcNAcΪԭ��ת���Ʊ�3A5AF���о�����������Һ����Ϊ�ܼ������߽������ܽ��ڷ�ӦҺ�У�Ϊ�������Ӧ������������Һ����۽ϸߡ��������Է�������⣬����ʵ�ֹ�ҵ��������̽�����Ӿ��á���ɫ����Ч���ܼ��ʹ�������δ��ʵ��3A5AF�Ĺ�ҵ����������Ҫ�о�����
����3������ת���Ʊ�3A5AF�о���չ
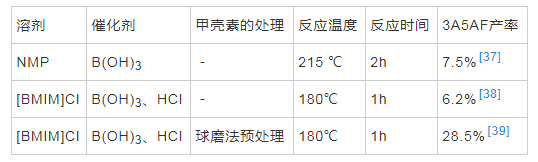
���������GlcNAc��ֱ���Լ�����Ϊԭ�Ͼ��и���ľ��þ����������Ǽ��ؽṹ���ܣ����и��ӵ�������磬���Խ�۲���һ����Ӧ����3A5AF��CHEN��[37]�״α�����ֱ���Լ���Ϊԭ���Ʊ� 3A5AF����N-��������ͪ��N-Methyl pyrrolidone��NMP����Ϊ�ܼ�������ͼ����Ȼ�����Ϊ��������215���·�Ӧ2h�����ص�ת���ʴﵽ50%������3A5AF�����ʽϵͣ����Ϊ7.5%������Һ����һ�ֶ��ǵ������ܼ���CHEN��[38]��10���־��в�ͬ�������Ӻ������ӵ�����Һ��Ϊ�ܼ���������ת��Ϊ3A5AF����[BMIM]Cl���������HCl��Ϊ������180���·�Ӧ1h��3A5AF����Ϊ6.2%����GlcNAc��Ϊ��Ӧ��ʱһ�����ڲ�ͬ������Һ���е�ʵ��Ҳ�����������ӵĴ��ڶԷ�Ӧ�нϴ��Ӱ�졣���⣬������Һ���ܼ��еķ�Ӧ�¶�Զ������NMP�ܼ���215��ķ�Ӧ�¶ȣ�����ѧ���о�������������Һ���У�3A5AF����10min��Ѹ���γ��ҷ�Ӧ����ʼ����Զ�������л��ܼ���[38]���������о�������������л��ܼ�NMP��˵������Һ���������3A5AF�IJ�����������Һ��ļ۸��Ϊ���������ڴ��ģ�Ĺ�ҵ�����������������о���Ӧ�ÿ�������������ɫ���õ��ܼ������ͳɱ������3A5AF�����ʡ�
�����ڼ���ת��Ϊ3A5AF��������Ӧ������û�м�GlcNAc��������÷�Ӧ�����ٲ����Ǽ��ص�ˮ�⣬������GlcNAcת����3A5AF�Ĺ��̡����⣬�о����֣����ŷ�Ӧʱ��Ľ�һ�������ӣ�3A5AF�����ʷ����½�������3A5AF�ڷ�Ӧ��ϵ���ֽ⣬���ȶ�[37]��Ϊ���ƻ����ص����ܽṹ��������磬�Լ��ؽ���Ԥ����ʹ����ٽ�ۣ�����併��ɵ��ǵ����ʣ��Ӷ����3A5AF�IJ��ʡ��о�����ĥ����ĥ�����������Ʒ�����������ᴦ��������Һ���ܽ���ٳ�����5�ֲ�ͬԤ���������������ضԷ�Ӧ��Ӱ�죬������ĥ�������������ƻ��侧��ṹ��������磬�ٽ����һ�����⣬3A5AF�IJ�����߿ɴﵽ28.5%[39]������ת��Ϊ3A5AF���ܵķ�Ӧ������ͼ4��ʾ�����ȣ�����ˮ���Ϊ���壬��������칹��Ϊ���ǣ�������ȥһ���ӵ�ˮ�γ���Ӧ��ͪ�����ѵ������ӵ�ˮ���γ�3A5AF[26]��
�������صĽṹ��������Ϊԭ���Ʊ�3A5AF�Դ��ڲ��ʵ͡��������������֮�������ڼ��ص�ˮ����������Ӧ�����ٲ��裬��ˣ�̽���ܽ��Ը�ǿ���ܼ��Լ���ͬ��Ԥ�����������ǽ��������3A5AF�IJ��ʵ�һ����Ҫ�о�����2�ܽ���Ŀǰ����ת��Ϊ3A5AF�ľ��弼������Ӧ�����
����ͼ4�����Ʊ�3A5AF�Ŀ��ܷ�Ӧ����[26]
![ͼ4�����Ʊ�3A5AF�Ŀ��ܷ�Ӧ����[26]](http://www.xueshut.com/uploads/allimg/200706/36-200F6105415R9.jpg)
����Fig. 4Possiblereaction process of conversion of chitin to 3A5AF[26]
������2 ����ת��Ϊ3A5AF�о�����ܽ�
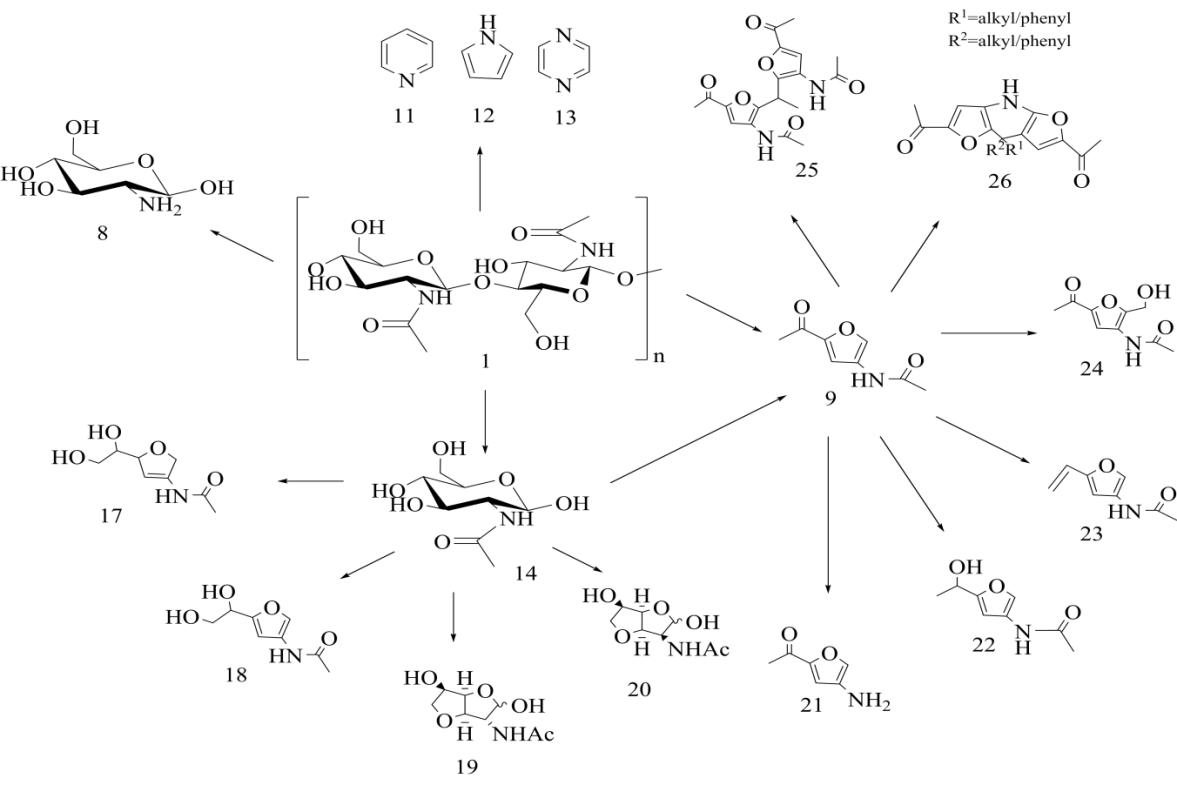
����3A5AF��Ϊһ�ֺ������ƽ̨������ת����һϵ�����λ����ͼ5������Щ���λ�������3-����-5-������ૣ�������21���ǿ������İ���������ͨ��3A5AF����ˮ���Ʊ��õ�����3A5AF��������ͨ����ת�Ƽ�������⻯�ƻ�ԭ���Ʊ���Ӧ�ĺ�������������22�����Ҳ��ʼ��ߣ����Դﵽ91%����������22���ʻ�����ˮ���γ���Ӧ�ĺ���ϩ����������23��������������£�3A5AF�뼸���ʻ������ﷴӦ�õ�һϵ�л����24��25�����Լ������ण�������26��[26]�����У������ा��н��ͼ����ļ����������ʣ�������Ȯ������֧ѭ��������[40]���Լ״�Ϊ�ܼ���������������Ϊ20%������������Һ��3A5AF����«ȩ��Ӧ�Ʊ�ϩͪ������ԼΪ58%[30]��3A5AF�����ԽϷ����ת��Ϊ뿣������Ҫ���м��壬��ͨ��Beckmann���ŷ�Ӧ����3,5-�������[41]��뿻�����Ҫ�ķ����Լ����綡��ͪ뿿����ڶ�ͪ��ȩ�ļ���ʵ�顣3A5AF��N-±���塢�⣩���������ǰ�����±����Ӧ���Եõ�2-��-3-����ૡ�2-��-3-����ૡ�2-��-3-����ૺ�2-��-3-������ǽ�Ϊ��Ҫ���м��壬����ͨ������ż����Ӧ��C2λ�ϰ�װC—C��C—����ԭ�Ӽ��Ӷ���һ���ϳ�һЩ����ҩ��[42,43]��3A5AF������������������������������½���Vilsmeier-Haack��Ӧ�����ɺ����Ŀ�ȩ������[44]����ȩ��������������Ҫ�Ļ���ԭ�ϣ���������֬�ĺϳɵ�����[45]��3A5AF��������Լױ�������ص��η�Ӧ�õ�2-����-3-����ૣ����ںϳ�ֲ������ҩ��[46]��ͨ��������Ӧ�����Խ���ȡ������ת�Ƶ�3A5AF��C2λ���ϣ��Ӷ�����2-�����[30]����������૿����صĹؼ��ǻ��������������Ϊ�ܼ���3A5AF��ż����������������40���·�Ӧ5h������������[30]�����º�2-��������Խ�һ����Ӧ�ϳ�2,3-������ૣ����Ǻ�����Ȼ���������Hyrtioseragamine�еĽṹ��Ԫ[47]�����⣬3A5AF��������Ϊҽҩ�м��壬�ϳɾ��п��������Ե�ProximicinA�ȣ����кܸߵľ��ü�ֵ��
��������3A5AF�ṹ��ƽ̨�ṹ���ԣ����Ŷ�3A5AF�о��IJ������룬δ�����и���ļ�����Դ�ĺ�����ѧƷ�õ��㷺���������ã���ҽҩ�������Ͳ��ϵ�������й�����Ӧ��ǰ����
����ͼ53A5AF�ϳɵ�������Ҫ����������[26]
![ͼ53A5AF�ϳɵ�������Ҫ����������[26]](http://www.xueshut.com/uploads/allimg/200706/36-200F6105333118.jpg)
����Fig.5N-containing compounds synthesized by 3A5AF
����5������ת���Ʊ���������������
��������3A5AF���⣬���ػ�����ת��Ϊ�����ĺ�����������ؾ���ˮ����Ҫת��Ϊ���������ǡ�GlcNAc���ǹ��ǵȺ����Ĺ��ǻ���[48]��AJAVAKOM��[49]ʹ�������ȴٽ�����ˮ�⣬��ʹ��ӦЧ�����10����FUKUOKA��[50]�Ƚ�������������д�������ʹ����ĥ����������ĥ6h����Ҫ���GlcNAc��PIERSON��[51]���֣��Դ���Ϊ����Һ�����ܼ���������������Ϊ8%��������Ϊ���������Ի�û����15�������һ�-2-��������-2-����������������ֲ�������ʿ��Դﵽ30%��
����CHEN��[34]��200���¶�GlcNAc�����Ƚ⣬���ֺ��������3A5AF�⣬������ມ���������वȲ��������ʽϵ͡�GAO��[2]��300���£���������NaOH��Һ�н����Ƚ⣬���������������������ຼ����������ȣ���������Ϊ��Ҫ�IJ�����о���̽���˲�ͬŨ�ȵ�NaOH�Է�Ӧ��Ӱ�죬������1mol/L��NaOH�з�Ӧ5min���Ի������������Ϊ2%���ڴ˻���������NH3����325���·�Ӧ5min����ʹ����������������12%������������������һ����Ҫ�ĺ������ӻ�������Ǻϳ�ҩ���Ȼ���P���ϵ���Ҫԭ��[52]����ຳ�����ҽҩ���м��壬���п������Ĺ��ܣ�Ҳ�ǰ��е���Ҫ��ζ������[53]��Ŀǰ��������������ʯ��Ϊԭ�ϣ��Ƚ�ʯ���ѽ�Ϊ1,3-����ϩ���ٽ���ת��Ϊૣ������백��Ӧ������������һ�����������ʿɴﵽ90%����ʯ��ת��Ϊ�������ܲ��ʽ�Ϊ3%����[2]�������Ŀǰ��ҵ�����������Ʊ�;����˵���Լ���Ϊԭ���Ʊ�������Ӧ���̸��Ӽ��ڽ����о��п�ͨ���������Դ������Ż���Ӧ�����ȷ�����һ������������IJ��ʣ�����Ϊ�����Ĺ�ҵ�������ṩ�µķ�����ͼ6չʾ���Լ���Ϊԭ��ת���Ʊ��ĸ��ֺ��������Ϊ���������ʵ������ṩ�˸���Ŀ��ܣ��ḻ�˺���������ĺϳɷ���������ᾭ�õķ�չ�����ش����塣
����ͼ6���ؿ�ת���Ʊ��ĸ��ֺ���������
����Fig.6Different N-containing compounds prepared from chitin
����6��������չ��
�������������Կ���������������Դ��Ϊԭ��������ѧƷ�õ���Խ��Խ������ӣ�����ȼ���Ҵ�������Ȼ�ѧƷ�������Ѿ�ʵ���˲�ҵ�����Խ��������Ⱦ����ʯ��Դ��ȱ������������Ҫ�����á�������Ϊȫ��ڶ���Ķ�����������Դ�����к��зḻ����Ȼ��Ԫ�أ��Լ��ؼ��䵥��GlcNAcΪԭ���Ʊ����ֺ���������ȡ���˽ϴ�Ľ�չ��������Ҫ�ľ��ú�����ֵ��
����Ŀǰ�����ؼ��䵥��ת���Ʊ�3A5AF�������������⣬���ڼ��ؽṹ�Ľᾧ�ȸߡ�����ѣ�����Ŀ��������ʽϵ͡�������ࡣ��������о����ھ��෴Ӧ����ϵ�н��У�������Ⱦ��Ϊ���ء���ʴ��ǿ��ȱ�㣬��GlcNAcΪԭ��ʱ��������ڼ��أ�����������ʵ�ֹ�ҵ����������Ȼ�����ߡ����⣬Ŀǰ�ķ�Ӧ��ϵ�л����ڴ��������ظ�ʹ�á������ѷ����ѡ���Խϵ͵����⡣�ڽ��������о������У�Ӧ���ص��о�����������ɫ�Ĵ��������������������ݷ�Ӧ����Ҫ���������Կɵ����Ի����ѺõĴ�����ͬʱʵ�ִ������ظ�ʹ�ã����ͳɱ������⣬������̽�����ִ����Ĺ���Ч��������߷�Ӧ��ѡ���Լ���������ʣ���ԭ�ϽǶ������������������ԭ�ϣ����Ǽ��ص����ܽ���Ӱ�췴ӦЧ�ʣ�����Һ����ܽ���أ���ʵ�������ת������������Һ��ļ۸��Ϊ���������о�Ӧ�ó��Կ��������۸�ϵ͡���ɫ�Ҿ��нϸ��ܽ��Ե��ܼ���һ���濪�����صĸ�Ч��ɫԤ����������һ���潨�������ھ�����ܽ�״̬�µķǾ����ת������������ؽ����е����ٲ���ķ�ӦЧ�ʣ���ʵ���乤ҵ�������Ĺؼ����裬ҲΪδ���Լ���Ϊԭ��ת���Ʊ�����ĺ�����ѧƷ�ṩ�˸���Ŀ��ܡ�
���������Ʊ����������ӻ�������ı��������٣������Ų��ʽϵͣ�������϶�����⡣���������о����Խ������Ŀ����������ӻ���������Ʊ��ϣ��Ż���Ӧ������̽������Ч�Ĵ���ϵ����߲�������ʣ��Ӷ�������ں�����ѧƷ���������Ӧ�÷�Χ��
���������
����[1] WANG Y X (��Ӣ��), HOU X L (������), ZHU Y L (������). The catalytic conversion of sugar derivatives to oxygen containing liquid fuel and fine chemicals[J]. Biotechnology & Business(�����ҵ����), 2017, (3): 48-55.
����[2] GAO X Y, CHEN X, ZHANG J G, et al. Transformation of chitin and waste shrimp shells into acetic acid and pyrrole[J]. ACS Sustainable Chemistry, 2016, 4(7): 3912-3920.
����[3] ZHANG M C (������),ZANG H J(갺鿡), YU S B (���ɰ�), et al. Advances in conversion of chitin biomass into high-value chemicals[J]. Chemical Industry and Engineering Progress(������չ), 2017, 36(3): 863-872
����[4] ZHAO H, HOLLADAY J E, BROWN H, et al. Metal chlorides in ionic liquid solvents convert sugars to 5-hydroxymethylfurfural[J]. Science, 2007, 316(5831): 1597-1600.
����[5] CHHEDA J N, ROMáN-LESHKOV Y, DUMESIC J A. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono-and poly-saccharides[J]. Green Chemistry, 2007, 9(4): 342-350.
����[6] GALLEZOT P. Conversion of biomass to selected chemical products[J]. Chemical Society Reviews, 2012, 41(4): 1538-1558.
����[7] BANERJEE R, CHINTAGUNTA A D, RAY S. A cleaner and eco-friendly bioprocess for enhancing reducing sugar production from pineapple leaf waste[J]. Journal of Cleaner Production, 2017, 149: 387-395.
����[8] HAYES D J, FITZPATRICK S, HAYES M H, et al. The biofine process–production of levulinic acid, furfural, and formic acid from lignocellulosic feedstocks[J]. Biorefineries–Industrial Processes, 2006, 1: 139-164.
����[9] SHI N (ʯ��), TANG W Y (������), TANG S Y (��ʯ��), et al. Advances in the catalytic conversion of lignocellulosic derived platform chemicals into liquid alkanes[J]. Chemical Industry and Engineering Progress(������չ), 2019, 38(7): 3097-3110.
����[10] XIE W J (л��ά), ZHANG X W(������), XIE J J (л����), et al.Synthesis of high-density jet fuels from biomass[J]. Progress in Chemistry(��ѧ��չ), 2018, 30(9): 1424-1433
����[11] WEI W(κޱ), CHANG F X (������), SUN J Z (�ィ��), et al. Recent advances in“one-pot”bioethanol production from lignocellulose[J]. Biomass Chemical Engineering(�����ʻ�ѧ����), 2018, 52(1): 53-59.
����[12] JIA W M (������),WANG Z J (��֮��),CHENG C Z (�̴���).Chemical conversions of biomass into aroma chemicalss—the use oflactic acid[J]. Fine Chemicals(��ϸ����), 2009, 26(12): 1167-1169, 1214.
����[13] KUANG B F(��̷�),JIANG T(����),YU Y L(������), et al.Research progress on hydrogenolysis of biomass to1,5-pentanediol and 1,6-hexanediol[J].Fine Chemicals(��ϸ����), 2019, 36,(5): 781-785.
����[14] OMARI K W, DODOT L, KERTON F M. A simple one�\pot dehydration process to convert N-acetyl-D-glucosamine into a nitrogen�\containing compound, 3-acetamido-5-acetylfuran[J]. ChemSusChem, 2012, 5(9): 1767-1772.
����[15] PHAM T T, CHEN X, YAN N, et al. A novel dihydrodifuropyridine scaffold derived from ketones and the chitin-derived heterocycle 3-acetamido-5-acetylfuran[J]. ChemistrySelect, 2018, 149(4): 857-861.
����[16] DOAN C T, TRAN T N, VO T P K, et al. Chitin extraction from shrimp waste by liquid fermentation using an alkaline protease-producing strain, Brevibacillus parabrevis[J]. International Journal of Biological Macromolecules, 2019, 131: 706-715.
����[17] PATIL N S, WAGHMARE S R, JADHAV J P . Purification and characterization of an extracellular antifungal chitinase from penicillium ochrochloron MTCC 517 and its application in protoplast formation[J]. Process Biochemistry, 2013,48(1): 176-183.
����[18] KURITA K. Controlled functionalization of the polysaccharide chitin[J]. Progress in Polymer Science, 2001, 26(9): 1921-1971.
����[19] KERTON F M, LIU Y, OMARI K W, et al. Green chemistry and the ocean-based biorefinery[J]. Green Chemistry, 2013, 15(4): 860-871.
����[20] LUCAS N, ATHAWALE A A, RODE C V. Valorization of oceanic waste biomass: A catalytic perspective[J]. The Chemical Record, 2019, 19(9): 1995-2021.
����[21] LIU Y (����), FANG G H (������), RONG S H (���غ�), et al. Research progress on the utilization of shrimp and crab shells[J]. Journal of Food Safety and Quality(ʳƷ��ȫ�������ѧ��), 2018, 9(3): 461-466.
����[22] WANG A Q (������). Chitin Chemistry[M]. Beijing: Science Press (��ѧ������)��2008: 9.
����[23] DU Y M (������). Research progress and application of chitin[J]. Fine and Specialty Chemicals(��ϸ��ר�û�ѧƷ), 2000, (14): 3-6.
����[24] CHEN X, YAN N. Novel catalytic systems to convert chitin and lignin into valuable chemicals[J]. Catalysis Surveys from Asia, 2014, 18(4): 164-176.
����[25] SHELDON R A. Green and sustainable manufacture of chemicals from biomass: state of the art[J]. Green Chemistry, 2014, 16(3): 950-963.
����[26] HüLSEY M J , ENVIRONMENT. Shell biorefinery: A comprehensive introduction[J]. Green Energy, 2018, 3(4): 318-327.
����[27] ZHU H, CAO Q, LI C H, et al. Acidic resin-catalysed conversion of fructose into furan derivatives in low boiling point solvents[J]. Carbohydrate Research, 2011, 346(13): 2016-2018.
����[28] OSADA M, KIKUTA K, YOSHIDA K, et al. Non-catalytic synthesis of Chromogen��and �� from N-acetyl-D-glucosamine in high-temperature water[J]. Green Chemistry, 2013, 15(10): 2960-2966.
����[29] LU X X, ZHENG Z S, ZHANG X, et al. New chromogen ketone compound for preparing medicament for the treatment or prevention of inosine-5-monophosphate dehydrogenase-mediated diseases in mammals, where diseases include immune rejection, autoimmune diseases, viral infections��CN110551095-A [P].2019-12-10.
����[30] PHAM T T, LINDSAY A C, KIM S W, et al. Two-Step preparation of diverse 3-Amidofurans from chitin[J]. ChemistrySelect, 2019, 4(34): 10097-10099.
����[31] WOLTER F E, MOLINARI L, SOCHER E R, et al. Synthesis and evaluation of a netropsin–proximicin-hybrid library for DNA binding and cytotoxicity[J]. Bioorganic, 2009, 19(14): 3811-3815.
����[32] PHAM T T, LINDSAY A C, CHEN X, et al. Transferring the biorenewable nitrogen present in chitin to several N-functional groups[J].Sustainable Chemistry and Pharmacy, 2019, 13: 100143.
����[33] FRANICH R A, GOODIN S J, WILKINS A L, et al. Acetamidofurans, acetamidopyrones, and acetamidoacetalde-hyde from pyrolysis of chitin and n-acetylglucosamine[J]. Journal of Analytical, 1984, 7(1/2): 91-100.
����[34] CHEN J H, WANG M F, HO C-T, et al. Volatile compounds generated from thermal degradation of N-acetylglucosamine[J]. Journal of Agricultural,1998, 46(8): 3207-3209.
����[35] DROVER M W, OMARI K W, MURPHY J N, et al. Formation of a renewable amide, 3-acetamido-5-acetylfuran, via direct conversion of N-acetyl-D-glucosamine[J]. RSC Advances, 2012, 2(11): 4642-4644.
����[36] WANG J, ZANG H J, JIAO S L, et al. Efficient conversion ofN-acetyl-D-glucosamine into nitrogen-containing compound 3-acetamido-5-acetylfuran using amino acid ionic liquid as the recyclable catalyst[J]. Science of The Total Environment, 2020, 710, DOI: https://doi.org/10.1016/j.scitotenv.2019.136293.
����[37] CHEN X, CHEW S L, KERTON F M, et al. Direct conversion of chitin into a N-containing furan derivative[J]. Green Chemistry, 2014, 16(4): 2204-2212.
����[38] CHEN X, LIU Y, KERTON F M, et al. Conversion of chitin and N-acetyl-D-glucosamine into a N-containing furan derivative in ioni liquids[J]. RSC Advances, 2015, 5(26): 20073-20080.
����[39] CHEN X, GAO Y J, WANG L, etal. Effect of treatment methods on chitin structure and its transformation into nitrogen-containing chemicals[J]. ChemPlusChem, 2015, 80(10): 1565-1572.
����[40] JIA M J(������), CHEN S L(��ʿ��), LIAO Y L (������), et al.Effects of furyl dihydropyriodine��on coronary collateral circu lation in dogs with acute myocardial infarction[J]. Acta Pharmacologica Sinica(�й�ҩ��ѧ��), 1994, (5): 80-83.
����[41] SU W K, ZHANG Y, LI J J, et al. A simple and efficient procedure for the beckmann rearrangement of oxime using bis-(trichloromethyl) Carbonate/DMF[J]. Organic Preparations and Procedures International, 2008, 40(6): 543-550.
����[42] ROY D, UOZUMI Y, CATALYSIS. Recent advances in palladium-catalyzed cross-coupling reactions at ppm to ppb molar catalyst loadings[J]. Advanced Synthesis, 2018, 360(4): 602-625.
����[43] FISCHER C, KOENIG B. Palladium-and copper-mediated N-aryl bond formation reactions for the synthesis of biological active compounds[J]. Beilstein Journal of Organic Chemistry, 2011, 7(1): 59-74.
����[44] CHEN S, WOJCIESZAK R, DUMEIGNIL F, et al. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural[J]. Chemical Reviews, 2018, 118(22): 11023-11117.
����[45] ZHOU C L (���ֳ�), LI Y F (�����), MEN X H (��ѧ��), et al. Research progress of functional polymer materials derived from furfural[J]. Journal of Functional Materials (���ܲ���) , 2005, (4): 499-502.
����[46] KOLODZIEJCZYK K, ROIBAN G D, SCHNüRCH M, et al. Synthesis of potential fungicides based on N-(3-furanyl) pyrrolecarboxamides and N-(3furanyl) pyrazolecarboxamid-es[J]. Monatshefte für Chemie Chemical Monthly, 2009, 140(11): 1349-1359.
����[47] TAKAHASHI Y, IINUMA Y, KUBOTA T, et al. Hyrtioseragamines A and B, New alkaloids from the sponge hyrtios species[J]. Organic Letters, 2011, 13(4): 628-631.
����[48] CHEN X, YANG H Y, YAN N. Shell biorefinery: dream or reality?[J]. Chemistry–A European Journal, 2016, 22(38): 13402-13421.
����[49] AJAVAKOM A, SUPSVETSON S, SOMBOOT A, et al. Products from microwave and ultrasonic wave assisted acid hydrolysis of chitin[J]. Carbohydrate Polymers, 2012, 90(1): 73-77.
����[50] YABUSHITA M, KOBAYASHI H, KUROKI K, et al. Catalytic depolymerization of chitin with retention of N-Acetyl group[J]. ChemSusChem, 2015, 8(22): 3760-3763.
����[51] PIERSON Y, CHEN X, BOBBINK F D, et al. Acid-catalyzed chitin liquefaction in ethylene glycol[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(8): 2081-2089.
����[52] OU Z P(ŷ��ƽ),WU Y Z(��Ԫ��),ZHOU F(�ܷ�), et al.Synth of five polysubstitued pyrroles[J]. Fine Chemicals(��ϸ����), 2009, 26(6): 609-612.
����[53] WANG Y F (���ȷ�), ZHOU R (����), ZHANG M C (������), et al.Research progress in important function pyrazine compounds in baijiu[J]. Liquor Making(���), 2018, 45(6): 20-24