
橙皮苷和橙皮素是柑橘类果皮的主要药效成分。近年的研究表明,二者在抗氧化和清除自由基、调节心血管系统功能、保护神经系统、抗过敏、抗皮炎、抗菌抗癌及基因毒性等方面表现出较好的生物活性。席夫碱指由含有活泼羰基的化合物与含氨基的化合物通过缩水形成的含碳氮双键的亚氨基(RHCRN)R或烷亚氨基(RRCRN)R类有机化合物。席夫碱中RCRNR双键上的RNR原子有孤对电子,具有重要的化学与生物学意义,近年来,对其在抗肿瘤、抗氧化和抗菌活性研究方面取得了很大进展。
自由基是生物体生命活动过程中由机体生物化学反应所产生的中间产物,对机体的衰老和疾病、正常的免疫代谢和细胞信号传导等过程均起着重要作用。研究发现生物体的多种疾病均与自由基对机体的氧化损伤有关。因此,开发能够清除自由基的抗氧化剂成为医药生物学研究的热点。
本文从中药陈皮中提取橙皮苷,酸水解制备橙皮素,然后分别与苯肼类、苯甲酰肼类、苯氨基脲类、苯氨基硫脲类、苯乙酰肼类、苯氧乙酰肼类和苯氨基乙酰肼类等含氨基化合物缩合反应得到橙皮素的18R个新席夫碱化合物,根据RUV、IR、1HRNMR、MSR图谱及元素分析对目标化合物的结构进行了表征,比较了橙皮苷、橙皮素和橙皮素席夫碱清除超氧自由基(RO-·2)R、羟自由基(·OH)R和(RDPPH·)R自由基的活性并测定了它们的总还原能力,为以橙皮黄酮为原料的新生物活性药物的开发和应用提供了实验数据。
1、R实验部分
1.R1R仪器和试剂
X-4R型显微熔点测定仪(R北京泰克公司)R,温度计未校正;RUV-2550R型紫外可见分光光度计(R日本岛津公司)R;RVarioRELRcubeR型元素分析仪(R德国RElementarR公司)R;RFT-IRR6700R型红外光谱仪(R美国RNicoletR公司)R,KBrR压片;RAvanceRAVR400MHzR超导核磁共振仪(R德国RBrukerR公司)R,溶剂为RDMSO-d6;RDSQR型质谱仪(R美国RThermoR公司)R。陈皮购自本地市场;R硅胶为青岛海洋化工厂生产;R二苯代苦味肼基自由基DPPH·为RSigmaR产品;R苯肼类、苯甲酰肼类、苯氨基脲类、苯氨基硫脲类、苯乙酰肼类、苯氧乙酰肼类和苯氨基乙酰肼类化合物均为自制;R其余试剂为国产化学纯或分析纯。
1.R2R橙皮素席夫碱衍生物的制备
橙皮苷的提取和分离纯化参考文献方法进行。所得橙皮苷为白色片状结晶,产率为R1.R3%,mp为R260R~262R℃,微溶于甲醇、乙醇,易溶于二甲亚砜、N,N-二甲基甲酰胺,其盐酸-镁粉实验为红色,1%AlCl3实验为黄色,10%KOHR实验为黄色。
橙皮素的制备参考文献方法进行。所得橙皮素为淡黄色针状结晶,产率为R52.R1%,mpR为R224R~226R℃R,不溶于石油醚,易溶于甲醇、乙醇,盐酸-镁粉实验为红色,1%RAlCl3实验为黄色,10%RKOHR实验为黄色。
橙皮素席夫碱衍生物的制备:R将R1RmmolR橙皮素和R1.R1RmmolR含氨基化合物溶于R20RmLR无水乙醇中并滴加少量冰醋酸或R1R滴浓盐酸,混合液搅拌回流R24R~60Rh,薄层层析检测反应进程,得棕黄色溶液,减压除去部分乙醇,有黄色固体析出,抽滤,蒸馏水洗涤固体,再用少量乙醇洗涤,室温晾干,无水乙醇/二甲亚砜重结晶,得到相应的橙皮素席夫碱类化合物。合成路线如RSchemeR1R所示。

1.R3R清除自由基活性实验
橙皮苷、橙皮素及其席夫碱类衍生物清除自由基活性试验参考文献方法进行,其中羟自由基由RFe2R+-H2O2-亚甲蓝体系产生,超氧自由基采用碱性条件下邻苯三酚的自氧化产生,DPPH·自由基直接用无水乙醇配制使用,还原能力测定采用铁氰化钾还原法。试样为浓度R1.R0Rg/LR的二甲亚砜溶液。
2、R结果与讨论
2.R1R橙皮素席夫碱衍生物的结构表征橙皮素对氯苯腙(R1a)R:R棕黄色粉末,产率R19.R7%,mpR232R~R234R℃,不溶于石油醚,易溶于甲醇、乙醇,盐酸-镁粉实验为红色,1%AlCl3实验为黄褐色,10%KOHR实验为黄褐色。UV(RDMSO)R,λmax/Rnm:R339,297;REI-MS(RmR/Rz,%R)R:R426(RM+,3.R36)R,411(R3.R12)R,300(R4.R25)R,179(R33.R17)R,137(R100)R,107(R19.R12)R,77(R24.R03)R;1HRNMR(RDMSO-d6)R,δ:R2.R72(Rdd,JR=R2.R80,17.R10RHz,1H,3-HRcis)R,3.R04(Rdd,JR=R12.R70,17.R09Hz,1H,3-HRtrans)R,3.R86(Rs,3H,4'-OCH3)R,5.R29(Rdd,JR=R2.R72,12.R52RHz,1H,2-H)R,5.R61(Rs,1H,3'-OH)R,5.R90(Rs,2H,6,8-H)R,6.R89R~R6.R95(Rm,3H,2',5',6'-H)R,7.R22R(Rd,JR=R8.R77RHz,2H,2″,6″-H)R,7.R57R(Rd,JR=8.R81RHz,2H,3″,5″-H)R,9.R38R(Rs,1H,7-OH)R,10.R05R(Rs,1H,—NH)R,12.R05R(Rs,1H,5-OH)R;RIRR(RKBr)R,σR/cm-R1:3497、3446(ROH)R,3326(RNH)R,2915,1608(RCRN)R,1587,1539,1515,1445;RC22H19ClN2O5元素分析(R计算值)R/%:RCR60.R03(R61.R90)R,HR4.R59(R4.R46)R,NR6.R71(R6.R57)R。
橙皮素对氯苯甲酰腙(R2a)R:R棕黄色粉末,产率R37.R8%,mpR246R~R248R℃,不溶于石油醚,易溶于甲醇、乙醇,盐酸-镁粉实验为红色,1%RAlCl3实验为黄褐色,10%RKOHR试验为黄褐色。UV(RDMSO)R,λmax/Rnm:R351,307;REI-MS(RmR/Rz,%R)R:R454(RM+,3.R04)R,436(R23.R45)R,297(R69.R16)R,185(R23.R79)R,139(R100)R,111(R32.R19)R,77(R9.R45)R;1HRNMR(RDMSO-d6)R,δ:R2.R77(Rdd,JR=2.R90,17.R08RHz,1H,3-HRcis)R,3.R07(Rdd,JR=12.R71,17.R09RHz,1H,3-HRtrans)R,3.R70R(Rs,3H,4'-OCH3)R,5.R32(Rdd,JR=R2.R75,12.R52RHz,1H,2-H)R,5.R64(Rs,1H,3'-OH)R,5.R89(Rs,2H,6,8-H)R,6.R92R~R7.R05(Rm,3H,2',5',6'-H)R,7.R24(Rd,JR=R8.R82RHz,2H,2″,6″-H)R,7.R59(Rd,JR=R8.R86RHz,2H,3″,5″-H)R,9.R40(Rs,1H,7-OH)R,10.R25(Rs,1H,—NH)R,12.R05(Rs,1H,5-OH)R;IR(RKBr)R,σR/Rcm-R1:R3495、3450(ROH)R,3331(RNH)R,2925,1610(RCRN)R,1543,1520,1465;RC23H19ClN2O6元素分析(R计算值)R/%:RCR59.R93(R60.R73)R,HR4.R39(R4.R18)R,NR6.R71(R6.R16)R。
橙皮素对羟基苯甲酰腙(R2b)R:R淡黄色粉末,产率42.R66%,mpR>300R℃。
1HRNMR(RDMSO-d6)R,δ:R2.R96(Rdd,JR=R5.R08,12.R16RHz,1H,3-HRcis)R,3.R42(Rdd,JR=R12.R04,17.R28RHz,1H,3-HRtrans)R,3.R78(Rs,3H,4'-OCH3)R,5.R10(Rdd,JR=R2.R68,9.R24RHz,1H,2-H)R,5.R64(Rs,1H,3'-OH)R,5.R90(Rs,1H,6-H)R,5.R93(Rs,1H,Ph—OH)R,5.R97R(Rs,1H,8-H)R,6.R84R~R7.R82R(Rm,7H,Ph—H)R,9.R42R(Rs,1H,7-OH)R,10.R99R(Rs,1H,—NH)R,13.R11(Rs,1H,5-OHR)R;RIRR(RKBrR)R,σR/Rcm-R1:R3508R(ROH)R,3339R(RNH)R,2939,2843,1654R(RCRO)R,1606(RCRN)R,1503,1480,1270,1173,1077,838。
橙皮素对硝基苯甲酰腙(R2c)R:R黄色粉末,产率R15.R05%,mpR>300R℃。
1HRNMR(RDMSO-d6)R,δ:R3.R07(Rdd,JR=R5.R24,11.R88RHz,1H,3-HRcis)R,3.R45(Rdd,JR=R12.R02,17.R32RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=R2.R84,8.R94RHz,1H,2-H)R,5.R69(Rs,1H,3'-OH)R,5.R91(Rs,1H,6-H)R,5.R93(Rs,1H,8-H)R,6.R86R~R7.R41R(Rm,7H,Ph—H)R,9.R07R(Rs,1H,7-OH)R,10.R26R(Rs,1H,—NH)R,12.R87R(Rs,1H,5-OH)R;RIR(RKBr)R,σ/cm-R1:R3378(ROH)R,3299(RNH)R,2938,2845,2641,1629(RCRO)R,1596(RCRN)R,1516,1461,1275,1156,1078,1023,835。
橙皮素间甲基苯甲酰腙(R2d)R:R黄色粉末,产率R15.R21%,mpR>R300R℃。
1HRNMR(RDMSO-d6)R,δ:R1.R07(Rs,3H,-CH3)R,3.R07(Rdd,JR=5.R24,11.R88RHz,1H,3-HRcis)R,3.R46(Rdd,JR=12.R70,17.R12RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=R2.R80,8.R8RHz,1H,2-H)R,5.R68(Rs,1H,3'-OH)R,5.R90(Rs,1H,6-H)R,5.R91(Rs,1H,8-H)R,6.R86R~R7.R35(Rm,7H,Ph—H)R,9.R12(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87(Rs,1H,5-OH)R;RIRR(RKBr)R,σR/Rcm-R1:R3300(ROH)R,3287(RNH)R,2961,2936,2841,1635(RCRO)R,1597(RCRNRN)R,1515,1462,1278,1157,1080,1019,813。
橙皮素苯氨基脲腙(R3a)R:R黄色粉末,产率R19.R77%,mpR>R300R℃。
1HRNMR(RDMSO-d6)R,δ:R3.R07(Rdd,JR=R4.R44,12.R32RHz,1H,3-HRcis)R,3.R30(Rdd,JR=R12.R71,17.R10RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=R2.R83,8.R90RHz,1H,2-H)R,5.R62(Rs,1H,3'-OH)R,5.R91(Rs,2H,6-H,8-H)R,6.R87R~R7.R50(Rm,8H,Ph‰H)R,8.R76(Rs,1H,—NH)R,9.R12(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87(Rs,1H,5-OH)R;RIR(RKBr)R,σR/cm-R1:R3405(ROH)R,3231(RNH)R,2956,2834,1858,1662(RCRO)R,1595(RCRN)R,1533,1516,1441,1386,1279,1156,1088,1028,823。
橙皮素对甲基苯氨基脲腙(R3b)R:R黄色粉末,产率16.R84%,mpR>300R℃。
1HRNMR(RDMSO-d6)R,δ:R1.R12(Rs,3H,—CH3)R,3.R07(Rdd,JR=R3.R45,12.R52RHz,1H,3-HRcis)R,3.R30(Rdd,JR=R12.R69,17.R08RHz,1H,3-Htrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=2.R91,9.R06RHz,1H,2-H)R,5.R71(Rs,1H,3'-OH)R,5.R91(Rs,2H,6-H,8-H)R,6.R86R~R7.R37R(Rm,7H,Ph—H)R,8.R64R(Rs,1H,—NH)R,9.R10R(Rs,1H,7-OH)R,10.R24R(Rs,1H,—NH)R,12.R87(Rs,1H,5-OHR)R;RIRR(RKBrR)R,σR/Rcm-R1:R3409R(ROH)R,3298、3225R(RNH)R,2917,1670R(RCRO)R,1600(RCRN)R,1551,1516,1451,1332,1243,822。
橙皮素对氯苯氨基脲腙(R3c)R:R黄色粉末,产率R21.R73%,mpR>R300R℃。EI-MS(Rm/z,%)R:R469(RM+,0.R36)R,211R(R7.R02R)R,185R(R14.R35R)R,153R(R100R)R,127R(R54.R32R)R,111R(R13.R12R)R,99R(R19.R88R)R,90R(R47.R31R)R,75(R17.R23)R,63(R32.R15)R,55(R9.R01)R;1HRNMR(RDMSO-d6)R,δ:R3.R07(Rdd,JR=5.R12,11.R92RHz,1H,3-HRcis)R,3.R44(Rdd,JR=12.R70,16.R96RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=2.R48,9.R00RHz,1H,2-H)R,5.R65(Rs,1H,3'-OH)R,5.R91(Rs,2H,6,8-H)R,6.R87R~R7.R55(Rm,7H,Ph—H)R,8.R79(Rs,1H,—NH)R,9.R11(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87R(Rs,1H,5-OH)R;RIRR(RKBr)R,,σR/Rcm-R1:R3397(ROH)R,3299、3224(RNH)R,2999,2958,1671R(RCROR)R,1596R(RCRNR)R,1536,1442,1360,1242,825;RC23H20ClN3O6元素分析(R计算值)R/%:RCR59.R03(R58.R79)R,HR4.R59(R4.R26)R,NR8.R71R(R8.R95)R。
橙皮素苯氨基硫脲腙(R4a)R:R黄色粉末,产率R21.R29%,mpR>R300R℃。
1HRNMRR(RDMSO-d6)R,δ:R3.R07(Rdd,JR=R5.R10,12.R21RHz,1H,3-HRcis)R,3.R41(Rdd,JR=R12.R69,17.R08RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R18(Rdd,JR=3.R03,9.R20RHz,1H,2-H)R,5.R71(Rs,1H,3'-OH)R,5.R91(Rs,2H,6-H,8-H)R,6.R87R~R7.R43(Rm,8H,Ph—H)R,8.R74(Rs,1H,—NH)R,9.R11(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87(Rs,1H,5-OH)R;RIR(RKBr)R,σ/cm-R1:R3410(ROH)R,3295、3221(RNH)R,2955,2835,1597(RCRN)R,1555,1500,1446,1242,1156,1087,757。
橙皮素对甲基苯氨基硫脲腙(R4b)R:R黄色粉末,产率R32.R26%,mpR>R300R℃。
1HRNMR(RDMSO-d6)R,δ:
1.R07(Rs,3H,CH3)R,3.R07(Rdd,JR=R5.R08,12.R13RHz,1H,3-HRcis)R,3.R40(Rdd,JR=R12.R71,17.R38RHz,1H,3-Htrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R20(Rdd,JR=2.R92,8.R94RHz,1H,2-H)R,5.R67(Rs,1H,3'-OH)R,5.R90(Rs,2H,6-H,8-H)R,6.R85R~R7.R34R(Rm,7H,Ph—H)R,8.R71R(Rs,1H,—NH)R,9.R11R(Rs,1H,7-OH)R,10.R24R(Rs,1H,—NH)R,12.R87(Rs,1H,5-OH)R;RIR(RKBr)R,σR/Rcm-R1:R3408(ROH)R,3229(RNH)R,2956,2834,1596(RCRN)R,1569,1532,1442,1278,1191(RCRSR)R,1088,823。
橙皮素对氯苯氨基硫脲腙(R4c)R:R黄色粉末,产率R32.R96%,mpR>R300R℃。EI-MS(Rm/z,%)R:R450(R13.R65)R,300(R62.R15)R,285(R33.R72)R,178(R100)R,150(R38.R24)R,137(R79.R05)R,107(R51.R23)R,77(R43.R55)R,55(R58.R03)R;1HRNMRR(RDMSO-d6)R,δ:R3.R07R(Rdd,JR=R5.R03,12.R12RHz,1H,3-HRcis)R,3.R42R(Rdd,JR=R12.R08,17.R26RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=2.R88,9.R70RHz,1H,2-H)R,5.R75(Rs,1H,3'-OH)R,5.R89(Rs,2H,6,8-H)R,6.R86R~R7.R31(Rm,7H,Ph—H)R,7.R89(Rs,1H,—NH)R,9.R11(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87R(Rs,1H,5-OH)R;RIRR(RKBr)R,σ/cm-R1:R3408R(ROH)R,3205R(RNH)R,2999,2956,1596(RCRNN)R,1568,1441,1243,823;RC23H20ClN3O5SR元素分析R(R计算值)R/R%R:RCR56.R42R(R56.R85R)R,HR4.R65(R4.R12)R,NR8.R95(R8.R65)R。
橙皮素苯乙酰腙(R5a)R:R黄色粉末,产率36.R41%,mpR>300R℃。EI-MS(Rm/z,%)R:R434(RM+,2.R97)R,300(R52.R66)R,285(R22.R59)R,150(R32.R63)R,137(R47.R30)R,107(R46.R97)R,91(R45.R50)R,77(R46.R20)R,55(R75.R99)R;1HRNMR(RDMSO-d6,400RMHz)R,δ:R3.R06R(Rdd,JR=R2.R87,17.R01RHz,1H,3-HRcis)R,3.R45R(Rdd,JR=R8.R18,11.R36RHz,1H,3-HRtrans)R,3.R62(Rs,2H,—CH2)R,3.R78(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=R2.R12,9.R43RHz,1H,2-H)R,5.R67(Rs,1H,3'-OH)R,5.R91(Rs,2H,6,8-H)R,6.R87R~R7.R28(Rm,8H,Ph—H)R,9.R10(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87R(Rs,1H,5-OH)R;RIRR(RKBr)R,σ/cm-R1:R3409R(ROH)R,3232R(RNH)R,2999,2956,1660(RCRO)R,1596(RCRN)R,1569,1532,1441,1387,1028,823;RC24H22N2O6元素分析(R计算值)R/%:RCR66.R17(R66.R36)R,HR5.R35(R5.R07)R,NR6.R72(R6.R45)R。
橙皮素对硝基苯乙酰腙(R5b)R:R黄色粉末,产率R25.R05%,mpR300R℃。
1HRNMR(RDMSO-d6,400RMHz)R,δ:R3.R07(Rdd,JR=R2.R65,16.R98RHz,1H,3-HRcis)R,3.R32(Rdd,JR=R7.R63,12.R11RHz,1H,3-HRtrans)R,3.R67(Rs,2H,—CH2)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=R2.R82,10.R23RHz,1H,2-H)R,5.R76(Rs,1H,3'-OH)R,5.R91(Rs,2H,6,8-H)R,6.R86R~R7.R21R(Rm,7H,Ph—H)R,9.R11R(Rs,1H,7-OH)R,10.R23R(Rs,1H,—NH)R,12.R86R(Rs,1H,5-OH)R;RIR(RKBr)R,σR/Rcm-R1:R3401(ROH)R,3297(RNH)R,2999,2956,2835,1665(RCRO)R,1595(RCRN)R,1568,1533,1442,1360,1087,824。
橙皮素萘乙酰腙(R5c)R:R黄色粉末,产率R17.R35%,mpR>R300R℃。
1HRNMRR(RDMSO-d6,400RMHz)R,δ:3.R07(Rdd,JR=R5.R16,11.R92RHz,1H,3-HRcis)R,3.R34R(Rs,2H,-CH2)R,3.R45(Rdd,JR=R12.R70,17.R12RHz,1H,3-Htrans)R,3.R79(Rs,3H,4'-OCH3)R,5.R19(Rdd,JR=2.R56,8.R96RHz,1H,2-H)R,5.R72(Rs,1H,3'-OH)R,5.R91(Rs,2H,6,8-H)R,6.R87R~R7.R52(Rm,10H,Ar—H)R,9.R11R(Rs,1H,7-OH)R,10.R24R(Rs,1H,—NH)R,12.R87R(Rs,1H,5-OH)R;RIR(RKBr)R,σ/cm-R1:R3399(ROH)R,3287(RNH)R,2956,1660(RCRO)R,1597(RCRN)R,1516,1443,1357,1243,1026,822。
橙皮素苯氧乙酰腙(R6a)R:R黄色粉末,产率R38.R8%,mpR>300R℃。EI-MS(Rm/z,%)R:R450(RM+,12.R37)R,314(R14.R08)R,300(R71.R96)R,285(R32.R23)R,178(R81.R33)R,150(R42.R09)R,137(R100)R,107(R33.R17)R,69(R80.R06)R,55(R57.R88)R;1HRNMR(RDMSO-d6,400RMHz)R,δ:R2.R50(Rdd,JR=R2.R90,17.R05RHz,1H,3-HRcis)R,3.R06(Rdd,JR=7.R88,11.R04RHz,1H,3-HRtrans)R,3.R31R(Rs,2H,—CH2O)R,3.R78R(Rs,3H,4'-OCH3)R,5.R18R(Rdd,JR=R2.R42,9.R24RHz,1H,2-H)R,5.R72(Rs,1H,3'-OH)R,5.R88(Rs,2H,6,8-H)R,6.R87R~R7.R03(Rm,3H,2',5',6'-H)R,6.R92(Rm,3H,Ar—H)R,6.R97R(Rm,2H,Ar—H)R,9.R06R(Rs,1H,7-OH)R,10.R21R(Rs,1H,—NH)R,12.R85R(Rs,1H,5-OH)R;RIR(RKBr)R,σ/cm-R1:R3404(ROH)R,3240(RNH)R,2997,2955,1664(RCRO)R,1596(RCRN)R,1532,1516,1440,1218,1028,824;RC24H22N2O7元素分析(R计算值)R/%:RCR63.R27(R63.R98)R,HR4.R53(R4.R89)R,NR6.R84(R6.R22)R。
橙皮素-(R2,4-二氯)R苯氧乙酰腙(R6b)R:R黄色粉末,产率R41.R22%,mpR>R300℃;1HRNMRR(RDMSO-d6,400RMHz)R,δ:R3.R07(Rdd,JR=R5.R16,11.R96RHz,1H,3-HRcis)R,3.R46(Rdd,JR=R12.R71,17.R70RHz,1H,3-HRtrans)R,3.R79(Rs,3H,4'-OCH3)R,4.R85(Rs,2H,—CH2O)R,5.R19R(Rdd,JR=R2.R76,8.R84RHz,1H,2-H)R,5.R63R(Rs,1H,3'-OH)R,5.R91(Rs,2H,6,8-H)R,6.R87R~R7.R61(Rm,6H,Ph—H)R,9.R11(Rs,1H,7-OH)R,10.R25(Rs,1H,—NH)R,12.R87(Rs,1H,5-OH)R;RIR(RKBr)R,σ/cm-R1:R3399(ROH)R,3221(RNH)R,2998,2956,1667(RCRO)R,1594(RCRN)R,1533,1442,1243,1027,823。
橙皮素苯氨基乙酰腙(R7a)R:R黄色粉末,产率R41.R43%,mpR>300R℃。
1HRNMR(RDMSO-d6,400RMHz)R,δ:R3.R06(Rdd,JR=R2.R81,17.R02RHz,1H,3-HRcis)R,3.R32R(Rdd,JR=R11.R27,16.R32RHz,1H,3-HRtrans)R,3.R68R(Rs,2H,—CH2)R,3.R79(Rs,3H,4'-OCH3)R,4.R33(Rs,1H,—NH)R,5.R20(Rdd,JR=R2.R98,12.R59RHz,1H,2-H)R,5.R70(Rs,1H,3'-OH)R,5.R90(Rs,2H,6,8-H)R,6.R86R~R7.R18(Rm,8H,Ph—H)R,9.R11(Rs,1H,7-OH)R,10.R24(Rs,1H,—NH)R,12.R87(Rs,1H,5-OH)R;RIR(RKBr)R,σ/cm-R1:R3407(ROH)R,3227(RNH)R,2998,2956,1660(RCRO)R,1596(RCRN)R,1516,1441,1260,1087,823。
橙皮素对甲基苯氨基乙酰腙(R7b)R:R黄色粉末,产率R18.R57%,mpR>R300R℃。EI-MS(Rm/z,%)R:R463(RM+,4.R09)R,434(R17.R06)R,312(R13.R07)R,300(R67.R10)R,285(R37.R87)R,178(R100)R,150(R49.R11)R,137(R91.R97)R,107(R61.R00)R,91(R42.R96)R,77(R53.R72)R,69(R67.R92)R,55(R38.R93)R;1HRNMR(RDMSO-d6,400RMHz)R,δ:R1.R50(Rs,3H,—CH3)R,3.R07(Rdd,JR=2.R85,17.R05RHz,1H,3-HRcis)R,3.R45(Rdd,JR=R12.R72,17.R10RHz,1H,3-HRtrans)R,3.R65(Rs,2H,—CH2)R,3.R78(Rs,3H,4'-OCH3)R,4.R30(Rs,1H,—NH)R,5.R18(Rdd,JR=R2.R72,12.R50RHz,1H,2-H)R,5.R69(Rs,1H,3'-OH)R,5.R90R(Rs,2H,6,8-H)R,6.R87R~R7.R13R(Rm,7H,Ph—H)R,9.R11R(Rs,1H,7-OH)R,10.R25(Rs,1H,—NH)R,12.R87R(Rs,1H,5-OH)R;RIRR(RKBr)R,σ/cm-R1:R3401R(ROH)R,3222R(RNH)R,2998,2955,1661(RCRO)R,1597R(RCRN)R,1532,1441,1243,1027,822;RC25H25N3O6元素分析(R计算值)R/%:RCR64.R32(R64.R80)R,HR5.R14(R5.R40)R,NR9.R25(R9.R07)R。
测定了橙皮苷、橙皮素和化合物R1a、2aR的紫外光谱,在紫外光谱中Ⅰ带(R300R~R400Rnm)R和Ⅱ带(R240R~285Rnm)R是黄酮类化合物的2R个主要吸收带,测定结果可以看出化合物1a、2aR的Ⅰ带及Ⅱ带均发生红移,证明形成了席夫碱。测定了橙皮苷、橙皮素和R18R个橙皮素席夫碱的红外光谱,从谱图中可以看到母体化合物的RCRO吸收峰消失,代之出现了席夫碱RCRN吸收峰。测定了化合物R1a、2a、3c、4c、5a、6aR和R7bR的质谱,从图中可以看到化合物的分子离子峰较弱,其它的离子峰可以得到较好的解释。测定了R18R个橙皮素席夫碱的核磁共振氢谱,能与结构较好吻合。对化合物R1a、2a、3c、4c、5a、6aR和R7bR进行了元素分析,结果与理论值能较好吻合。
通过化合物的紫外吸收光谱、红外光谱、1HRNMRR和RMSR图谱,可以确定生成的衍生物是目标化合物。
2.R2R化合物的抗氧化活性
近年来,人们普遍认为生命过程中氧化代谢反应产生的各种过量自由基与许多疾病,如肿瘤、炎症和衰老等密切相关,因此寻找低毒或无毒的抗氧化物质已成为热点研究课题。本文测定了橙皮苷、橙皮素及席夫碱新化合物清除超氧自由基(RO-·2)R、羟自由基(·OH)R和R2,2-二苯基-1-苦味酰基自由基(RDPPH·)R的活性及总还原能力。实验过程中选用了多个浓度,结果发现低浓度时,清除自由基效果较弱,而高浓度时化合物在测定体系中的溶解度小,清除自由基效果也较弱,下面比较了浓度为R1.R0Rg/L时各化合物的抗氧化活性,结果列于表R1。
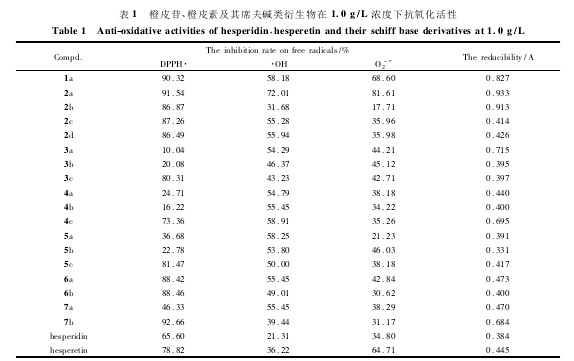
从表R1R可以看出,在R1.R0Rg/LR时,橙皮苷、橙皮素及其席夫碱新化合物均具有清除自由基活性,并且部分席夫碱化合物作用强于橙皮苷和橙皮素,其中橙皮素对甲基苯氨基乙酰腙(R化合物R7b)R对RDPPH·自由基清除活性较强,清除率为R92.R66%;R橙皮素对氯苯甲酰腙(R化合物R2a)R清除羟自由基(·OH)R及超氧自由基(RO-·2)R作用均较强,清除率分别为R72.R01%R和R81.R61%;R橙皮素对氯苯甲酰腙(R化合物R2a)R总还原能力较强,其抗氧化活性均强于橙皮苷和橙皮素,具体规律和作用机理尚待进一步研究。
3、R结R论
从中药陈皮中提取精制橙皮苷,通过酸水解制备橙皮素,再分别与R7R种类型的含氨基化合物在酸催化下缩合反应得到18R个相应的橙皮素席夫碱衍生物,用RUV、IR、1HRNMR、MSR及元素分析等技术手段对结构进行了表征,比较了橙皮苷、橙皮素和这些橙皮素席夫碱衍生物清除超氧自由基(RO-·2)R、羟自由基(·OH)R和RDPPH·自由基的活性。结果表明,橙皮苷、橙皮素和橙皮素席夫碱衍生物均具有清除自由基活性,并且部分席夫碱化合物作用强于橙皮苷和橙皮素,其中橙皮素对甲基苯氨基乙酰腙(R化合物R7b)R对DPPH·自由基清除活性较强,橙皮素对氯苯甲酰腙R(R化合物R2a)R对羟自由基R(R·OH)R和超氧自由基(RO-·2)R清除作用及总还原能力均较强,值得进一步研究开发。
参 考 文 献
[1]Choi E M,Kim Y H. Hesperetin Attenuates the Highly Reducing Sugar-triggered Inhibition of Osteoblast Differentiation[J]. Cell Biol Toxicol,2008,24( 3) : 225-231.
[2]Orallo F,Alvarez E,Basaram H,et al. Comparative Study of the Vasorelaxant Activity,Superoxide-scavenging Ability and Cyclic Nucleotide Phosphodiesterase-inhibitory Effects of Hesperetin and Hesperidin[J]. Naunyn Schmiedebergs ArchPharmacol,2004,370( 6) : 452-463.
[3]Kumar P,Kumar A. Protective Effect of Hesperidin and Naringin Against 3-Nitropropionic Acid Induced Huntington's Like Symptoms in Rats: Possible Role of Nitric Oxide[J]. Behav Brain Res,2010,206( 1) : 38-46.
[4]Fujita T,Shiura T,Masuda M,et al. Anti-allergic Effect of a Combination of Citrus Unshiu unripe Fruits Extract and Prednisolone on Picryl Chloride-induced Contact Dermatitis in Mice[J]. J Nat Med,2008,62( 2) : 202-206.
[5]Aranganathan S,Selvam J P,Sangeetha N,et al. Modulatory Efficacy of Hesperitin( Citrus Flavanone) on Xenobiotic-metabolizing Enzymes During 1,2-Dimethylhydrazine-induced Colon Carcinogenesis [J]. Chem-Biol Interact,2009,180( 2) : 254-261.
[6]Mazymdar A K. Synthesis of Thiazole Schiff Bases[J]. J Am Chem Soc,1979,56: 999.
[7]Nyarku S K,Mavso. Preparation,Charaterisation and Biological Evaluation of a Chromium( Ⅱ) Schiff Bases[J]. Orient J Chem,2000,16( 3) : 533-556.
[8]ZHU Yuchang,ZHOU Dazhai,WANG Cheng. Extraction of Hesperidin from Sweet Orange Peels[J]. Food Sci,2010,31( 22) : 264-267( in Chinese) .
朱玉昌,周大寨,王成. 甜橙皮中橙皮苷提取工艺[J]. 食品科学,2010,31( 22) : 264-267.
[9]SHAN Yang,LI Gaoyang,WANG Qiuan ,et al. Semisynthesis of Five Bioactive Flavonoids from Hesperidin[J]. Chinese J Org Chem,2008,28( 6) : 1024-1028( in Chinese) .
单杨,李高阳,汪秋安,等. 橙皮苷半合成 5 种生物活性黄酮类化合物[J]. 有机化学,2008,28( 6) : 1024-1028.
[10]Zhao Fang,Liang Hui,Cheng Hui,et al. Synthesis,Characterization and Antioxidative Activity of Metal Complexes with Rhein[J]. Acta Chim Sin,2011,69( 8) : 925-930( in Chinese) .
赵芳,梁慧,程惠,等. 大黄酸金属配合物的合成、表征及抗氧化活性研究[J]. 化学学报,2011,69( 8) : 925-930.