摘 要: 乳酸菌属于肠道内的有益菌,对调节人体健康有益,其生长能力和活性受外界条件的影响。促生长因子是一类对微生物生长繁殖具有促进作用的物质,可用于有效提高乳酸菌数量和活性。围绕乳酸菌促生长因子与益生元的关系及其来源、类别和作用机理等方面,综述其在乳酸菌上的研究与应用,并对未来发展方向进行了展望,以期为促生长因子在乳酸菌产业中的应用提供参考。
关键词: 乳酸菌; 促生长因子; 益生元; 作用机制;
Abstract: Lactic acid bacteria are beneficial bacteria in the intestinal tract, which is beneficial to the regulation of human health. Its growth ability and activity are affected by external conditions. Growth promoting-factor is a kind of substance which can promote the growth and reproduction of microorganism and can be used to effectively increase the quantity and activity of lactic acid bacteria. In this paper, we review the relationship between lactic acid bacteria growth promoting-factors and prebiotics, as well as their sources, classes and mechanisms of action, and look forward to their future development, in order to provide a reference for the application of growth-promoting factors in the lactic acid bacteria industry.
Keyword: lactic acid bacteria; growth promoting factor; prebiotics; mechanism;
乳酸菌(lactic acid bacteria)是一类革兰氏染色呈阳性的兼性厌氧细菌,在形态和生理特征方面不完全相同,能代谢多种碳水化合物并产生乳酸[1,2]。乳酸菌作为胃肠道中的“土着菌群”,是保证机体正常获取营养和维持健康的关键。目前,部分乳酸菌已被证明在实际生产应用中具有抗氧化、调节血糖血脂、降低胆固醇、缓解抑郁及自闭、改善乳糖不耐症和防治腹泻等多重益生功效[3,4,5,6,7,8,9]。

乳酸菌对生长条件的要求比较苛刻,在高浓度胃酸和胆盐以及各种消化酶的影响下容易丧失活性,甚至死亡;另一方面,乳酸菌容易受到宿主的饮食习惯、疾病、压力和衰老等诸多因素的影响,难以在结肠中长期定殖。近年来,为了保证乳酸菌食品在加工、储运过程中以及被机体摄入后始终保持较高的活菌数,越来越多的研究聚焦于寻找发酵过程中能促进乳酸菌生长繁殖和活性稳定的物质上[10,11,12]。促生长因子(growth-promoting factors)是一类对微生物生长繁殖具有促进作用的物质,可用于有效提高乳酸菌的数量和活性[13]。本文针对促生长因子与益生元(prebiotics)的关系及其来源、类别和作用机理等方面进行综述,旨在为促生长因子的开发利用提供理论参考。
1、 促生长因子与益生元的关系
促生长因子又叫促生长物质、增殖因子,是一类能促进乳酸菌生长繁殖且在结构和性质方面不完全相同的物质,可用于促进体外发酵体系中乳酸菌的增殖,也可用于提高人体中乳酸菌的丰度[13,14]。
益生元是指一类能被宿主体内有益微生物选择性利用,并对宿主机体产生正健康效应的基质[15]。作为一种无生命活性的饮食成分,益生元主要包括果聚糖(菊粉和低聚果糖)、低聚半乳糖和乳果糖;此外,低聚木糖、乳酮糖、抗性淀粉、膳食纤维和母乳低聚糖等也可被归类为新型益生元或潜在益生元[16,17,18]。目前,益生元主要从植物原料中提取,并通过酶促水解反应或者单糖及二糖的反式糖基化反应获得。它们能在进食后短时间内有效促进肠道蠕动,改变机体肠道菌群的组成[19]。国际上公认的益生元评判标准包括以下三点[20,21]:1)该物质能抵抗胃酸和各类消化酶,同时不被机体消化吸收;2)该物质可以被肠道菌群发酵利用;3)该物质可以选择性刺激肠道内有益菌的生长和/或活性。也就是说,只有完全满足以上评判标准的物质才可以被定义为益生元,才能声明其益生元效应。
能促进乳酸菌生长繁殖的任何一种膳食物质都可被定义为促生长因子。能到达结肠并被有益菌选择利用的任何一种促生长因子都是一种潜在的益生元[22]。根据促生长因子和益生元的定义以及评判标准可知,益生元和促生长因子在本质上都对乳酸菌的生长繁殖具有促进作用,因此可以认为益生元属于促生长因子的范畴,但促生长因子不一定是益生元。促生长因子只有在具有促进乳酸菌生长作用的同时,又可完全满足益生元的评判标准,才能被归属于益生元的范畴。归纳来说,所有的益生元都属于促生长因子,而促生长因子则是潜在的益生元。促生长因子与益生元的关系详见图1。
2、 促生长因子的作用评价
促生长因子可以缩短乳酸菌到达增殖稳定期的时间。促生长因子成分复杂,含有多种单糖和氨基酸组分,这些组分往往容易被乳酸菌分解。相比于培养基中的基础碳源和氮源,促生长因子可为乳酸菌的生长提供更优的碳氮组合。当培养基中含有促生长因子时,乳酸菌能够更快速有效地利用培养基中的营养物质,从而提前到达增殖稳定期,降低培养成本[23]。
促生长因子可以提高乳酸菌的菌群丰度。促生长因子中可能存在大分子的多糖(polysaccharides),它们的存在能为整个培养体系提供持久性的可发酵碳源。乳酸菌在消耗完体系中的基础碳源之后能继续获得营养补充,从而延长增殖稳定期的时间,促使发酵后期菌群丰度相对上升[16]。
图1 促生长因子与益生元的关系
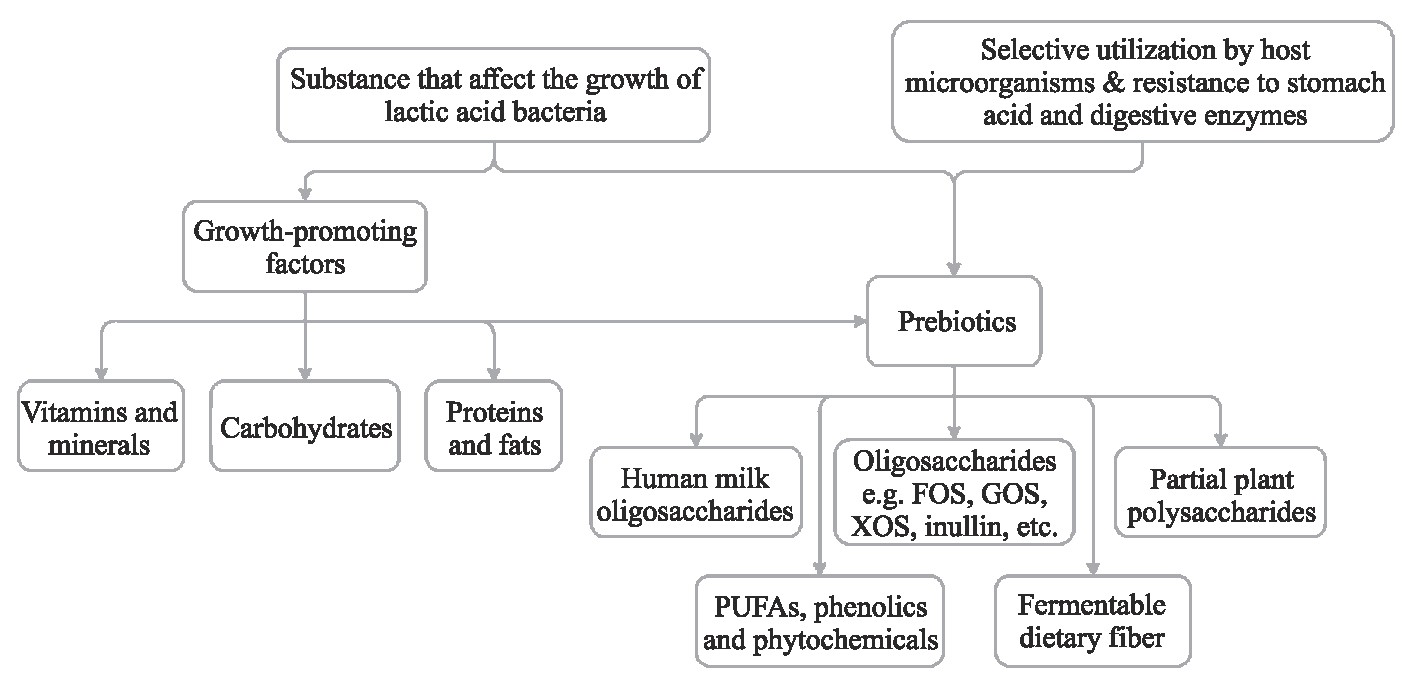
Fig.1 The relationship between growth-promoting factors and prebiotics
图中显示了潜在及公认的促生长因子和益生菌,相关文献证据水平各不相同。PUFA:多不饱和脂肪酸;FOS:果寡糖;GOS:半乳寡糖;XOS:木寡糖。
The figure shows candidate as well as accepted growth-promoting factors and prebiotics in which levels of reference evidence currently vary.PUFA:Polyunsaturated fatty acid;FOS:Fructooligosaccharides;GOS:Galactooligosaccharides;XOS:Xylooligosaccharide.
促生长因子可以促进乳酸菌产生更多的短链脂肪酸[24]。乳酸菌将低聚糖(oligosaccharides)、非淀粉多糖和抗性淀粉等不可消化的碳水化合物作为营养物质加以利用时,会生成大量的短链脂肪酸。短链脂肪酸的产生能够提供菌群生长代谢所需要的营养和适宜的p H值;能有效促进胃肠道蠕动、增加粪便湿度和排便效率,以便携带更多有害菌排出体外;同时,还能有效抑制病原菌的生长,促进机体对微量元素的吸收[25]。
总体来说,目前关于乳酸菌促生长因子的筛选评价主要通过观察乳酸菌能否快速地代谢该物质,并在此条件下能否获得较高的菌群丰度。但是此类方法的局限性在于试验所选择的乳酸菌不能真正代表结肠区的微生态系统,无法验证底物的选择性代谢,只能用于促生长因子的初步筛选。
3 、促生长因子的来源与分类
根据目前国内外的研究报道,乳酸菌促生长因子主要由以下5类物质组成:天然植物提取物类、糖类、蛋白质类、其他植物活性成分类和代谢产物类。
3.1、 天然植物提取物
天然植物提取物是一类以天然植物为原料,采用适当的溶剂和方法定向提取和浓缩其有效成分而得到的物质。其成分组成比较复杂,除了碳水化合物、蛋白质、有机酸和微量元素等基础营养物质外,还包含多酚、多糖、皂苷等多种生物活性成分[26]。部分天然植物提取物对乳酸菌增殖的影响详见表1。
3.2、 糖类物质
低聚糖是由2~10个单糖残基通过糖苷键连接而成的低聚合度糖类化合物。刘露等[34,35]采用淮山低聚糖取代葡萄糖作为碳源,发现嗜酸乳杆菌、嗜热链球菌和双歧杆菌的活菌数均有所提高;同时低聚糖的加入能提高双歧杆菌的生长速率,使其提前进入增殖稳定期。董会娟等[36]研究发现,添加魔芋低聚糖后,植物乳杆菌的活性和耐贮性得到了显着提高,存活率可达97%,较不添加时提高了13%。Lee等[37]采用β-葡萄糖寡糖作为唯一碳源进行发酵,发现其能显着促进乳酸菌的生长,可使乳酸链球菌肽的产量增长25%。
表1 部分天然植物提取物对乳酸菌增殖的影响
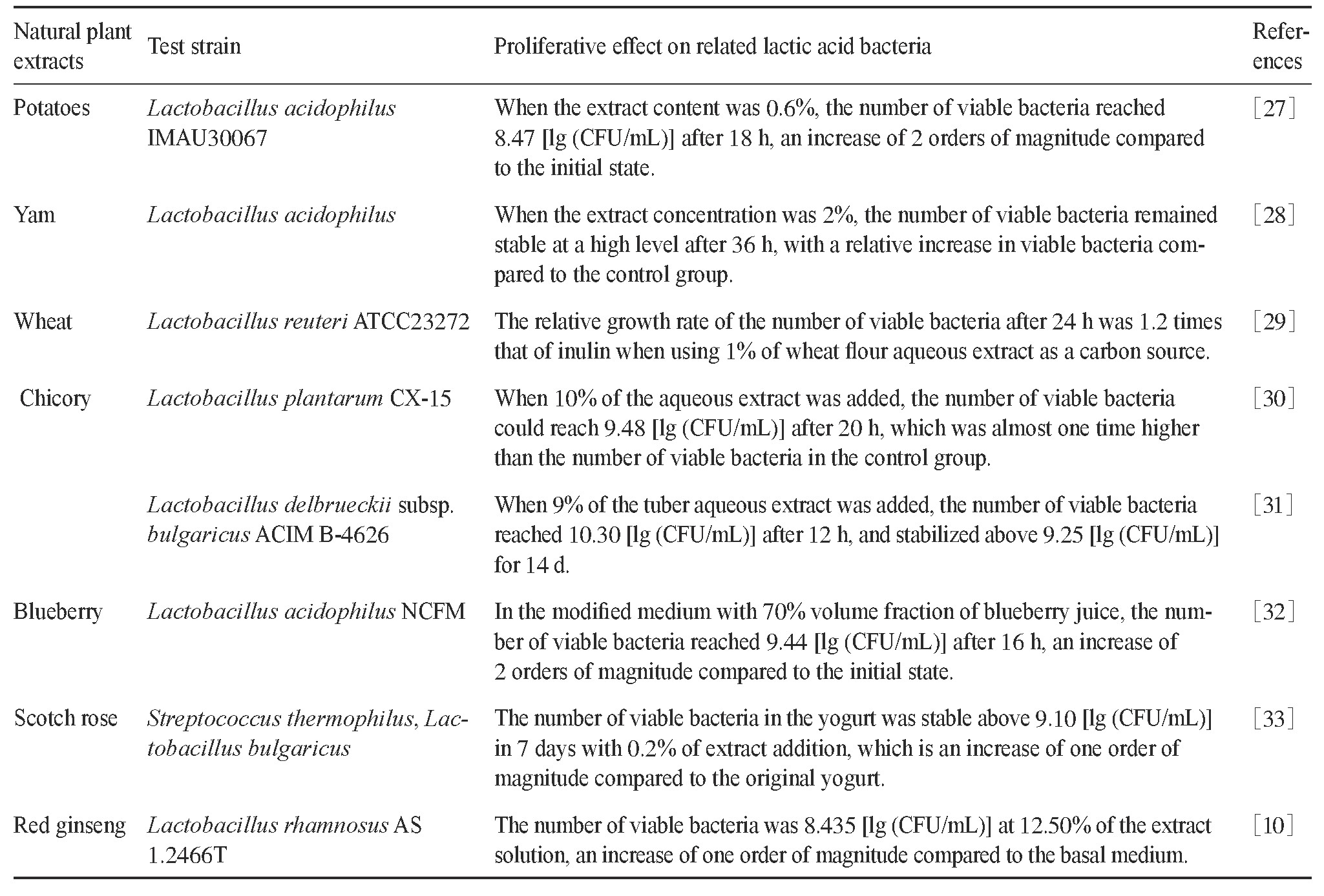
多糖是指10个或10个以上的单糖分子相连接而成的高分子聚合物,包含均多糖、杂多糖和糖蛋白[38]。多糖一般结构复杂、聚合度高,在被消化吸收之前必须经过相关酶的水解作用转化成可利用的低聚糖或单糖[39]。Sarikaya等[40]研究发现,菊粉在盲肠和直肠微生物悬浮液中厌氧发酵24 h后,乳杆菌、双歧杆菌和总细菌的数量明显增加,而有害代谢产物减少。郭羽等[41]针对黄芪多糖对鼠李糖乳杆菌的促生长作用进行了初步探究,得出黄芪多糖对鼠李糖乳杆菌的增殖具有一定的效果,还可提高其生长稳定性和对生长环境中不良因素的耐受性。林珊[42]研究发现,莲子抗性淀粉及其两个组分对婴儿双歧杆菌在内的6株双歧杆菌的生长均有促进作用,并且其组分被双歧杆菌发酵后,颗粒表面粗糙程度增加,出现片状结构。除此之外,苹果多糖、褐藻多糖、枸杞多糖和平菇多糖等也被证明具有促进乳酸菌生长的效果[12,43,44,45,46]。
糖醇(sugar alcohol)是指糖的还原性羰基通过加氢反应后生成的一类多元醇[47]。人体肠道内缺乏水解糖醇的相关酶系,进入小肠后无法被胃和小肠消化,从而进入大肠被乳酸菌利用[25]。胥媛媛等[48]研究发现,木糖醇能显着提高肠道菌群中双歧杆菌等有益菌的占比,同时发酵过程中短链脂肪酸的总量也迅速上升。Haukioja等[49]研究了不同乳酸菌在含有山梨醇的培养基中的产酸情况,发现鼠李糖乳杆菌能迅速利用山梨醇进行生长繁殖,并迅速将培养体系的p H值降低到5.0以下。
3.3 、蛋白质类
蛋白质及其水解产物的氮元素含量高,通常被用作微生物培养基的基础氮源。促生长因子含有比较丰富的有效氮源,可能有利于乳酸菌转运蛋白的合成,以提高乳酸菌对营养物质的转运效率,从而对乳酸菌的增殖作用更明显[50]。已有研究证实,蛋白质类促生长因子的共有成分为含硫肽,其中二硫键是保持其促生长活性的关键[51]。在MRS培养基中添加1.0 mg/m L的芝麻多肽能够显着促进保加利亚乳杆菌和嗜热链球菌的生长[52]。潘芬等[53]研究了豌豆蛋白酶解产物对多株乳酸菌生长的影响,结果表明,豌豆蛋白酶解产物可以促进干酪乳杆菌在内的4株乳酸菌的生长,其活菌数均可提高一个数量级以上。孙文敬等[50]研究发现,小麦蛋白胨含有的非游离氨基酸大分子量肽段有利于干酪乳杆菌等乳酸菌的生长繁殖。
3.4 、其他植物活性成分
已有研究表明,除了多糖和蛋白质以外,多酚、皂苷等植物活性成分也可以促进乳酸菌的生长[54]。Huang等[55]通过小鼠体内试验,研究了淮山薯蓣皂苷元对肠道菌群的影响,结果显示,薯蓣皂苷元对鼠乳杆菌和罗伊氏乳杆菌的生长具有显着的促进作用。同时,其构效关系分析表明,薯蓣皂苷元对乳酸菌的促生长作用依赖于其C5-C6的双键结构和完整的E-环和F-环。陈樱萌等[56]研究发现,添加0.1%槲皮素能促进酸奶发酵过程中乳杆菌和双歧杆菌的生长,并可抑制肠杆菌和肠球菌的生长。
3.5 、代谢产物类
代谢产物是指菌类在正常生长代谢过程中产生的物质,如细菌素、胞外多糖和短链脂肪酸等[57]。Gaikwad等[58]研究发现,地衣代谢物能提高干酪乳杆菌的干物质含量至56.08 mg,较对照组提高了24.49 mg(试验证明干物质量与细菌数量呈正相关关系)。黄蓉等[59]研究发现,以瑞士乳杆菌MB2-1总胞外多糖和胞外多糖-2纯组分为培养基中唯一碳源时,不仅能促进乳酸菌的生长,还能在一定程度上抑制有害菌的繁殖。
4 、促生长因子的作用机理
乳酸菌可通过分泌相关酶将促生长因子分解为基础营养物质,以促进其生长繁殖与代谢。相关的分解代谢主要包括两种方式:一是由转运蛋白将促生长因子完整地转运至细胞内,然后通过胞内的相关酶系对其进行糖酵解和磷酸化后加以利用,如嗜酸乳杆菌利用低聚果糖,双歧杆菌利用低聚木糖;二是先由胞外酶在细胞外对其水解,再由转运蛋白将水解产物运输至胞内继续水解,或者直接参与代谢,如罗伊氏乳酸菌代谢棉籽糖,嗜酸乳杆菌代谢低聚异麦芽糖[60,61]。两种方式各有利弊,前者局限于转运蛋白的转运能力,无法很好地利用大分子物质,后者则容易在胞外被其他菌群利用,不利于有益菌的选择性增殖。
天然植物提取物中的有机酸、生物碱等成分以及糖类和蛋白质类促生长因子在发酵过程中产生的短链脂肪酸能有效降低培养体系的p H值[28]。适宜的p H值有助于乳酸菌维持较好的黏附能力,直接利于其定殖和生长;同时培养体系中p H值的降低也会抑制有害菌的生长,间接利于乳酸菌的生长繁殖[25]。
体内培养时,促生长因子对于肠道有害菌有抑制和清除作用,能在一定程度上为肠道有益菌的生长繁殖提供更充足的生长空间和营养物质,从而间接达到促生长的效果[54,62]。促生长因子对于有害菌的影响途径主要包括:与有害菌的细胞膜或糖蛋白进行特异性结合,从而干扰其在肠道内的正常生长与定殖;通过产生H2O2等抑菌物质破坏细胞膜的通透性;通过干扰细菌群体感应中信号分子的合成,从而影响细胞膜的形成;与金属离子螯合生成不溶性复合物,从而抑制酶的活性等[63,64]。
5 、总结与展望
促生长因子不能依靠简单碳氮源自行合成,需要从外界摄取,对微生物的生长繁殖具有促进作用。其与益生元在定义和评价标准等方面既有区别也有相同之处。所有的益生元都属于促生长因子,而促生长因子可能成为潜在的益生元。促生长因子能在发酵过程中促进乳酸菌的生长繁殖和活性稳定,不受限于外部条件的影响,在乳酸菌相关产品生产中有较好的应用前景。
随着相关研究的不断深入,未来关于乳酸菌促生长因子还有以下几个方面值得深入研究和探讨:1)扩大对促生长因子来源的筛选,以期得到更多低成本、高产高效的促生长因子;2)不断优化各类促生长因子的分离纯化与制备方法,从而利于其加工应用和长期保存;3)进一步探索促生长因子发挥作用的量效、构效关系和多种促生长因子间的协同效应,同时考虑不同宿主的个性化差异,科学合理地选择乳酸菌-促生长因子组合,以期达到最佳的益生效果;4)采用混合培养的方式模拟肠道微生态,研究相关微生物菌群的数量变化,更好地评价其发酵的选择性;5)制定严格规范的评判标准,更加科学地使用促生长因子。
参考文献
[1]孟祥晨,杜鹏,李艾黎,等.乳酸菌与乳品发酵剂[M].北京:科学出版社, 2009:1-4.MENG Xiangchen, DU Peng, LI Aili, et al. Lactic acid bacteria and dairy starter culture[M]. Beijing:Science Press, 2009:1-4.
[2]张和平.自然发酵乳制品中乳酸菌生物多样性[M].北京:科学出版社, 2012:1.ZHANG Heping. Biopersity of lactic acid bacteria in naturally fermented dairy products[M]. Beijing:Science Press, 2012:1.
[3] QIAN F, ZHANG J, HOU K, et al. In vitro study of the antioxidative and antiproliferative capabilities of Lactobacillus casei16-fermented soymilk[J]. Food Science&Nutrition, 2019, 8(2):48-57.
[4] HSIEH M C, TSAI W H, JHENG Y P, et al. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus:a randomized, double-blinded, placebocontrolled trial[J]. Scientific Reports, 2018, 8(1):16791.
[5] MICHAEL D R, DAVIES T S, MOSS J W E, et al. The anti-cholesterolaemic effect of a consortium of probiotics:an acute study in C57BL/6J mice[J]. Scientific Reports, 2017, 7(1):2883.
[6] BABU C S, SUNANDA T, ABID B, et al. Autism and gut-brain axis:role of probiotics[J]. Advances in Neurobiology, 2020, 24:587-600.
[7] PAMER E G. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens[J]. Science, 2016, 352(6285):535-538.
[8] VITELLIO P, CELANO G, BONFRATE L, et al. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on gut microbiota in patients with lactose intolerance and persisting functional gastrointestinal symptoms:a randomised, double-blind,cross-over study[J]. Nutrients, 2019, 11(4):886.
[9] LI Y T, XU H, YE J Z, et al. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea:a systematic review with meta-analysis[J]. World Journal of Gastroenterology,2019, 25(33):4999-5016.
[10] 邹美娟,孙永胜,李晨,等.红参对乳酸菌体外增菌的影响[J].中国食品学报, 2020, 20(5):36-42.ZOU Meijuan, SUN Yongsheng, LI Chen, et al. Effects of red ginseng on the proliferation of lactic acid bacteria in vitro[J]. Journal of Chinese Institute of Food Science and Technology, 2020,20(5):36-42.
[11] 石梦玄,张璐,田美玲,等.基于体外模拟肠道微生态体系比较不同果蔬全粉的益生元功效[J].中国食品学报, 2020,20(2):87-94.SHI Mengxuan, ZHANG Lu, TIAN Meiling, et al. The comparison of prebiotic roles of different vegetable and fruit powders using in vitro simulation intestinal microecology system[J]. Journal of Chinese Institute of Food Science and Technology, 2020, 20(2):87-94
[12] LI Y, WANG S, SUN Y, et al. Apple polysaccharide could promote the growth of Bifidobacterium longum[J]. International Journal of Biological Macromolecules, 2020, 152:1186-1193.
[13]孟涛,郭兴华.乳酸菌及其生长因子对人畜健康的作用[J].生物工程进展, 1993, 13(4):48-52.MENG Tao, GUO Xinghua. The role of lactic acid bacteria and their growth factors in human and animal health[J]. China Biotechnology, 1993, 13(4):48-52.
[14] 鲁庆,鲁海波,刘香荣.益生菌增殖因子及其效果检验方法的研究进展[J].食品研究与开发, 2014, 35(16):132-136.LU Qing, LU Haibo, LIU Xiangrong, et al. Research advance on the multiplication things of probiotics[J]. Food Research and Development, 2014, 35(16):132-136.
[15] GIBSON G R, HUTKINS R, SANDERS M E, et al. Expert consensus document:The International Scientific Association for Probiotics and Prebiotics(ISAPP)consensus statement on the definition and scope of prebiotics[J]. Nature Review Gastroenterology&Hepatology, 2017, 14(8):491-502.
[16] GIBSON G R, RASTALL R A. Prebiotics:development&application[M]. Chichester:John Wiley&Sons, 2006:113-126.
[17] RENGADU D, GERRANO A S, MELLEM J J. Prebiotic effect of resistant starch from Vigna unguiculata(L.)Walp.(cowpea)using an in vitro simulated digestion model[J]. International Journal of Food Science and Technology, 2020, 55(1):332-339.
[18] PRAVEEN M A, PARVATHY K R K, JAYABALAN R, et al. Dietary fiber from Indian edible seaweeds and its in-vitro prebiotic effect on the gut microbiota[J]. Food Hydrocolloids, 2019, 96:343-353.
[19] SAAD N, DELATTRE C, URDACI M, et al. An overview of the last advances in probiotic and prebiotic field[J]. LWT-Food Science and Technology, 2013, 50(1):1-16.
[20] GIBSON G R, SCOTT K P, RASTALL R A, et al. Dietary prebiotics:current status and new definition[J]. Food Science&Technology Bulletin Functuinal Foods, 2010, 7(1):1-19.
[21] DAVANI-DAVARI D, NEGAHDARIPOUR M, KARIMZADEH I,et al. Prebiotics:definition, types, sources, mechanisms, and clinical applications[J]. Foods, 2019, 8(3):92.
[22] COLLINS M D, GIBSON G R. Probiotics, prebiotics, and synbiotics:approaches for modulating the microbial ecology of the gut[J]. The American Journal of Clinical Nutrition, 1999, 69(5):1052S-1057S.
[23] MONTAGNE L, PLUSKE J R, HAMPSON D J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals[J]. Animal Feed Science and Technology, 2003, 108(1/4):95-117.
[24] VERBEKE K A, BOOBIS A R, CHIODINI A, et al. Towards microbial fermentation metabolites as markers for health benefits of prebiotics[J]. Nutrition Research Reviews, 2015, 28(1):42-66.
[25] 刘松珍,张雁,张名位,等.肠道短链脂肪酸产生机制及生理功能的研究进展[J].广东农业科学, 2013, 40(11):99-103.LIU Songzhen, ZHANG Yan, ZHANG Mingwei, et al. Research progress on producing mechanism and physiological functions of intestinal short chain fatty acids[J]. Guangdong Agricultural Sciences, 2013, 40(11):99-103.
[26] COWAN M M. Plant products as antimicrobial agents[J]. Clinical Microbiology Reviews, 1999, 12(4):564-582.
[27] 赵燕霞,王元弛,姚国强,等.嗜酸乳杆菌IMAU30067增殖培养基的研究[J].中国乳品工业, 2019, 47(2):15-21.ZHAO Yanxia, WANG Yuanchi, YAO Guoqiang, et al. Study on the optimization of enrichment medium of Lactobacillus acidophilus IMAU30067[J]. China Dairy Industry, 2019, 47(2):15-21.
[28] LEE S Y, GANESAN P, AHN J, et al. Lactobacillus acidophilus fermented yam(Dioscorea opposita Thunb.)and its preventive effects on gastric lesion[J]. Food Science and Biotechnology,2011, 20(4):927-932.
[29] PAESANI C, SALVUCCI E, MOIRAGHI M, et al. Arabinoxylan from Argentinian whole wheat flour promote the growth of Lactobacillus reuteri and Bifidobacterium breve[J]. Letters in Applied Microbiology, 2019, 68(2):142-148.
[30] 程新,董英,苏萍,等.以菊芋为主要营养基质的乳酸菌发酵及影响因素[J].食品与发酵工业, 2013, 39(11):91-96.CHENG Xin, DONG Ying, SU Ping, et al. Cultivation and influencing factors of Lactobacillus plantarum CX-15 in Jerusalem artichoke tuber extract without acidic or enzymatic inulin hydrolysis[J]. Food and Fermentation Industries, 2013, 39(11):91-96
[31] PLOTNIKOVA V E, KARETKIN B A, PANFILOV V I. Jerusalem artichoke tubers for producing vegetable probiotic functional beverages with lactic acid bacteria[J]. IOP Conference Series:Earth and Environmental Science, 2020, 548(8):082075.
[32] 刘奕炜,周方,郝建新,等.蓝莓汁及其提取物对嗜酸乳杆菌NCFM体外生长的影响[J].中国乳品工业, 2013, 41(2):13-16.LIU Yiwei, ZHOU Fang, HAO Jianxin, et al. Supplement of blueberry juice and its extracts to improve the growth of Lactobacillus acidophilus NCFM as probiotic adjunct[J]. China Dairy Industry, 2013, 41(2):13-16.
[33] SZOTYSIK M, KUCHARSKA A Z, SOK?-TOWSKA A, et al.The effect of Rosa spinosissima fruits extract on lactic acid bacteria growth and other yoghurt parameters[J]. Foods, 2020, 9(9):1167.
[34] 刘露,张雁,邓媛元,等.山药低聚糖体外调节乳酸菌生长的作用[J].中国食品学报, 2017, 17(10):37-43.LIU Lu, ZHANG Yan, DENG Yuanyuan, et al. Regulation functions to Lactobacillus in vitro by oligosaccharides from Chinese yam[J]. Journal of Chinese Institute of Food Science and Technology, 2017, 17(10):37-43.
[35] 刘露,张雁,池建伟,等.山药低聚糖对2种双歧杆菌的体外促生长作用[J].食品科学, 2017, 38(6):130-135.LIU Lu, ZHANG Yan, CHI Jianwei, et al. Promoting effects of oligosaccharides from Chinese yam on the growth of two species of Bifidobacteria in vitro[J]. Food Science, 2017, 38(6):130-135.
[36] 董会娟,杨星月,周中凯.魔芋低聚糖对植物乳杆菌在加工过程中活性的影响[J].粮食与油脂, 2019, 32(6):82-86.DONG Huijuan, YANG Xingyue, ZHOU Zhongkai, et al. Effects of konjac oligosaccharides on the viability of Lactobacillus plantarum during drying process[J]. Cereals&Oils, 2019, 32(6):82-86.
[37] LEE J M, JANG W J, LEE E W, et al.β-glucooligosaccharides derived from barleyβ-glucan promote growth of lactic acid bacteria and enhance nisin Z secretion by Lactococcus lactis[J]. LWTFood Science and Technology, 2020, 122:109014.
[38] 聂少平,唐炜,殷军艺,等.食源性多糖结构和生理功能研究概述[J].中国食品学报, 2018, 18(12):1-12.NIE Shaoping, TANG Wei, YIN Junyi, et al. Research progress on structure and functional activities of food-derived polysaccharides[J]. Journal of Chinese Institute of Food Science and Technology,2018, 18(12):1-12.
[39] ZHANG T H, YANG Y, LIANG Y, et al. Beneficial effect of intestinal fermentation of natural polysaccharides[J]. Nutrients, 2018,10(8):1055.
[40] SARIKAYA H, ASLIM B, YUKSEKDAG Z. Assessment of antibiofilm activity and bifidogenic growth stimulator(BGS)effect of lyophilized exopolysaccharides(l-EPSs)from Lactobacilli strains[J]. International Journal of Food Properties, 2016, 20(2):362-371.
[41] 郭羽,王晓.黄芪多糖对鼠李糖乳杆菌促生长作用初探[J].山西中医学院学报, 2018, 19(4):10-14.GUO Yu, WANG Xiao. The growth-promoting effect of Astragalus polysaccharides on Lactobacillus rhamnosus[J]. Journal of Shanxi University of Chinese Medicine, 2018, 19(4):10-14.
[42]林姗.莲子抗性淀粉分级分离及其益生元作用的研究[D].福州:福建农林大学, 2016.LIN Shan. Grading alcohol precipitation of resistant starch derived from lotus seed, and its probiotics effects[D]. Fuzhou:Fujian Agriculture and Forestry University, 2016.
[43] ZHOU F, JIANG X Y, WANG T, et al. Lycium barbarum polysaccharide(LBP):a novel prebiotics candidate for Bifidobacterium and Lactobacillus[J]. Frontiers in Microbiology, 2018, 9:1034.
[44] KONG Q, DONG S, GAO J, et al. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota[J]. International Journal of Biological Macromolecules, 2016, 91:867-871.
[45] BAJURY D M, RAWI M H, SAZALI I H, et al. Prebiotic evaluation of red seaweed(Kappaphycus alvarezii)using in vitro colon model[J]. International Journal of Food Science and Nutrition,2017, 68(7):821-828.
[46] 郭芸,李海涛,赵玉明,等.富硒平菇多糖对益生菌生长代谢的研究[J].中国酿造, 2019, 38(11):145-148.GUO Yun, LI Haitao, ZHAO Yuming, et al. Effect of seleniumenriched Pleurotus ostreatus polysaccharides on growth and metabolism of probiotics[J]. China Brewing, 2019, 38(11):145-148.
[47]王凯,高群玉.糖醇食品健康营养新概念[J].粮油食品科技,2010, 18(1):42-46.WANG Kai, GAO Qunyu. New concept of sugar alcohol foods in health and nutrition[J]. Science and Technology of Cereals, Oils and Foods, 2010, 18(1):42-46.
[48] 胥媛媛,陈怡,葛茵,等.体外模拟结肠环境探究木糖醇对肠道菌群的影响[C].青岛:中国食品科学技术学会第十五届年会, 2018:155-156.XU Yuanyuan, CHEN Yi, GE Yin, et al. Effect of xylitol on the gut micribiota in an in vitro colonic stimulation[C]. Qingdao:The 15th Annual Conference of Chinese Society for Food Science and Technology, 2018:155-156.
[49] HAUKIOJA A, SODERLING E, TENOVUO J. Acid production from sugars and sugar alcohols by probiotic Lactobacilli and Bifidobacteria in vitro[J]. Caries Research, 2008, 42(6):449-453.
[50] 孙文敬,辛晓亚,郭文杰,等.小麦蛋白胨促进乳酸菌增殖的研究[J].食品与发酵工业, 2017, 43(3):66-72.SUN Wenjin, XIN Xiaoya, GUO Wenjie, et al. Promoting growth of four lactic acid bacteria by using prepared wheat peptones[J].Food and Fermentation Industries, 2017, 43(3):66-72.
[51] 孙纪录,贾英民,田洪涛,等.双歧杆菌生长促进因子的研究进展[J].食品工业科技, 2003, 24(8):110-112.SUN Jilu, JIA Yingmin, TIAN Hongtao, et al. Research progress of Bifidobacterium growth-promoting factors[J]. Science and Technology of Food Industry, 2003, 24(8):110-112.
[52] 钱森和,杨超英,薛正莲,等.芝麻多肽的特性及其抗氧化活性对乳酸菌生长影响的研究[J].中国油脂, 2018, 43(10):41-45.QIAN Senhe, YANG Chaoying, XUE Zhenglian, et al. Characteristics of sesame polypeptide and effects of its antioxidant activity on growth of lactic acid bacteria[J]. China Oils and Fats, 2018,43(10):41-45.
[53] 潘芬,杨敏,刘梦阳,等.豌豆蛋白酶解产物促进益生菌生长活性研究[J].中国食品学报, 2019, 19(2):27-36.PAN Fen, YANG Min, LIU Mengyang, et al. Growth-stimulating effects of pea protein hydrolysates on probiotics[J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(2):27-36.
[54]杨华,叶发银,赵国华.膳食多酚与肠道微生物相互作用研究进展[J].食品科学, 2015, 36(3):223-227.YANG Hua, YE Fayin, ZHAO Guohua. Advances in interactions between gut microflora and dietary polyphenols[J]. Food Science, 2015, 36(3):223-227.
[55] HUANG C H, CHENG J Y, DENG M C, et al. Prebiotic effect of diosgenin, an immunoactive steroidal sapogenin of the Chinese yam[J]. Food Chemistry, 2012, 132(1):428-432.
[56] 陈樱萌,苏立杰,丁静华,等.槲皮素酸奶的研制及其对小鼠肠道菌群的调节作用[J].现代食品科技, 2019, 35(12):189-196, 275.CHEN Yingmeng, SU Lijie, DING Jinghua, et al. Preparation of quercetin-fortified yogurt and its intestinal flora-regulating effect in mice[J]. Modern Food Science and Technology, 2019, 35(12):189-196, 275.
[57] ROOKS M G, GARRETT W S. Gut microbiota, metabolites and host immunity[J]. Nature Reviews Immunology, 2016, 16(6):341.
[58] GAIKWAD S, VERMA N, SHARMA B O, et al. Growth promoting effects of some lichen metabolites on probiotic bacteria[J]. Journal of Food Science and Technology, 2014, 51(10):2624-2631.
[59]黄蓉,张学亮,韩烁,等.瑞士乳杆菌MB2-1源胞外多糖对163-169.of exopolysaccharides of Lactobacillus helveticus MB2-1 on growth characteristics of ten probiotics[J]. Food Science, 2020,41(6):163-169.
[60] 陈臣,卢艳青,于海燕,等.乳酸菌代谢低聚糖机理的研究进展[J].中国食品学报, 2019, 19(6):274-283.CHEN Chen, LU Yanqing, YU Haiyan, et al. The research progress on mediating oligosaccharides metabolism by lactic acid bacteria[J]. Journal of Chinese Institute of Food Science and Technology, 2019, 19(6):274-283.
[61] TEIXEIRA J S, MCNEILL V, GANZLE M G. Levansucrase and sucrose phoshorylase contribute to raffinose, stachyose, and verbascose metabolism by Lactobacilli[J]. Food Microbiology,2012, 31(2):278-284.
[62] YAMAKOSHI J, TOKUTAKE S, KIKUCHI M, et al. Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora and faecal odor[J]. Microbial Ecology in Health and Disease, 2001, 13(1):25-31.
[63] MOHAMADZADEH M, OLSON S, KALINA W V, et al. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization[J]. Proceedings of the National Academy2880-2885.
[64] SIRK T W, FRIEDMAN M, BROWN E F. Molecular binding of black tea theaflavins to biological membranes:relationship to bioactivities[J]. Journal of Agricultural and Food Chemistry, 2011,59(8):3780-3787.
乳酸菌可以促进蛋白质、钙、铁等营养物质的吸收,有利于促进肠道益生菌的增殖,抑制有害菌的生长,能改善人体免疫力和提高抵抗力,有预防肠癌、保护肝脏、美容养颜等作用。...
近年来,国内外有不少关于产蛋白质类抑菌物质乳酸菌的研究报道,如LGG(Lactobacillus rhamnosus GG)、ZJ19[19]和KCA386[20],对李斯特氏菌、枯草芽孢杆菌和多数乳酸菌有抑制作用。...